Insight Into Interactions of L-Arginine/L-Histidine with Drug Betaine Hydrochloride in Aqueous Medium at Different Temperatures by Using Physicochemical Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amino Acid Recognition by Aminoacyl-Trna Synthetases
www.nature.com/scientificreports OPEN The structural basis of the genetic code: amino acid recognition by aminoacyl‑tRNA synthetases Florian Kaiser1,2,4*, Sarah Krautwurst3,4, Sebastian Salentin1, V. Joachim Haupt1,2, Christoph Leberecht3, Sebastian Bittrich3, Dirk Labudde3 & Michael Schroeder1 Storage and directed transfer of information is the key requirement for the development of life. Yet any information stored on our genes is useless without its correct interpretation. The genetic code defnes the rule set to decode this information. Aminoacyl-tRNA synthetases are at the heart of this process. We extensively characterize how these enzymes distinguish all natural amino acids based on the computational analysis of crystallographic structure data. The results of this meta-analysis show that the correct read-out of genetic information is a delicate interplay between the composition of the binding site, non-covalent interactions, error correction mechanisms, and steric efects. One of the most profound open questions in biology is how the genetic code was established. While proteins are encoded by nucleic acid blueprints, decoding this information in turn requires proteins. Te emergence of this self-referencing system poses a chicken-or-egg dilemma and its origin is still heavily debated 1,2. Aminoacyl-tRNA synthetases (aaRSs) implement the correct assignment of amino acids to their codons and are thus inherently connected to the emergence of genetic coding. Tese enzymes link tRNA molecules with their amino acid cargo and are consequently vital for protein biosynthesis. Beside the correct recognition of tRNA features3, highly specifc non-covalent interactions in the binding sites of aaRSs are required to correctly detect the designated amino acid4–7 and to prevent errors in biosynthesis5,8. -

Convenient Preparation of Poly(L-Histidine) by the Direct Polymerization of L-Histidine Or Nim Benzyl-L-Histidine with Diphenylphosphoryl Azide
Polymer Journal, Vol. 13, No. 12, pp 1151-1154 (1981) SHORT COMMUNICATION Convenient Preparation of Poly(L-histidine) by the Direct Polymerization of L-Histidine or Nim_Benzyl-L-histidine with Diphenylphosphoryl Azide Takumi NARUSE, Bun-ichiro NAKAJIMA, Akihiro TSUTSUMI, and Norio NISHI Department of Polymer Science, Faculty of Science, Hokkaido University, Nishi 8-chome, Kita 10-jo, Kita-ku, Sapporo 060, Japan. (Received August 14, 1981) KEY WORDS Polymerization I Diphenylphosphoryl Azide (DPPA) I L- Histidine I N'm-Benzyl-L-histidine I Poly(L-histidine) I Poly(N'm-benzyl-L histidine) I IR Spectrum I 13C NMR Spectrum I Intrinsic Viscosity I Poly(L-histidine) is a very interesting poly(a histidine) by the polymerization of L-histidine with amino acid) as a synthetic functional polymer or as iodine-phosphonic acid esters was unsuccessful. The a model for the functional biopolymer such as simplification of the synthetic route for poly(L enzymes. It is known, however, that poly(L histidine) is still necessary. histidine) can not be prepared by the Fuchs Diphenylphosphoryl azide (DPPA) has been used Farthing method 1 which is very popular for prepar as a convenient reagent for racemization-free pep ing poly(a-amino acid)s. A synthetic route with tide synthesis, since it was synthesized by Shioiri et several steps involving the synthesis of NCA (a a!. in 1972.6 Recently, we reported that this reagent amino acid N-carboxyanhydride) by the rather can also be used successfully for the polymerization classical Leuchs method/ have been used for the of amino acids or peptides. -

Amino Acids Amino Acids
Amino Acids Amino Acids What Are Amino Acids? Essential Amino Acids Non Essential Amino Acids Amino acids are the building blocks of proteins; proteins are made of amino acids. Isoleucine Arginine (conditional) When you ingest a protein your body breaks it down into the individual aminos, Leucine Glutamine (conditional) reorders them, re-folds them, and turns them into whatever is needed by the body at Lysine Tyrosine (conditional) that time. From only 20 amino acids, the body is able to make thousands of unique proteins with different functions. Methionine Cysteine (conditional) Phenylalanine Glycine (conditional) Threonine Proline (conditional) Did You Know? Tryptophan Serine (conditional) Valine Ornithine (conditional) There are 20 different types of amino acids that can be combined to make a protein. Each protein consists of 50 to 2,000 amino acids that are connected together in a specific Histidine* Alanine sequence. The sequence of the amino acids determines each protein’s unique structure Asparagine and its specific function in the body. Asparate Popular Amino Acid Supplements How Do They Benefit Our Health? Acetyl L- Carnitine: As part of its role in supporting L-Lysine: L-Lysine, an essential amino acid, is mental function, Acetyl L-Carnitine may help needed to support proper growth and bone Proteins (amino acids) are needed by your body to maintain muscles, bones, blood, as support memory, attention span and mental development. It can also support immune function. well as create enzymes, neurotransmitters and antibodies, as well as transport and performance. store molecules. N-Acetyl Cysteine: N-Acetyl Cysteine (NAC) is a L-Arginine: L-Arginine is a nonessential amino acid form of the amino acid cysteine. -

Solutions to 7.012 Problem Set 1
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Solutions to 7.012 Problem Set 1 Question 1 Bob, a student taking 7.012, looks at a long-standing puddle outside his dorm window. Curious as to what was growing in the cloudy water, he takes a sample to his TA, Brad Student. He wanted to know whether the organisms in the sample were prokaryotic or eukaryotic. a) Give an example of a prokaryotic and a eukaryotic organism. Prokaryotic: Eukaryotic: All bacteria Yeast, fungi, any animial or plant b) Using a light microscope, how could he tell the difference between a prokaryotic organism and a eukaryotic one? The resolution of the light microscope would allow you to see if the cell had a true nucleus or organelles. A cell with a true nucleus and organelles would be eukaryotic. You could also determine size, but that may not be sufficient to establish whether a cell is prokaryotic or eukaryotic. c) What additional differences exist between prokaryotic and eukaryotic organisms? Any answer from above also fine here. In addition, prokaryotic and eukaryotic organisms differ at the DNA level. Eukaryotes have more complex genomes than prokaryotes do. Question 2 A new startup company hires you to help with their product development. Your task is to find a protein that interacts with a polysaccharide. a) You find a large protein that has a single binding site for the polysaccharide cellulose. Which amino acids might you expect to find in the binding pocket of the protein? What is the strongest type of interaction possible between these amino acids and the cellulose? Cellulose is a polymer of glucose and as such has many free hydroxyl groups. -
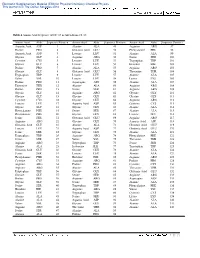
Table 2 Amino Acid Sequence of OC-17 As Taken from Ref. 28 Amino
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics This journal is © The Owner Societies 2012 Table 2 Amino Acid Sequence of OC-17 as taken from ref. 28 Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Aspartic Acid ASP 1 Alanine ALA 49 Arginine ARG 97 Proline PRO 2 Glutamic Acid GLU 50 Phenyalanine PHE 98 Aspartic Acid ASP 3 Leucine LEU 51 Alanine ALA 99 Glycine GLY 4 Arginine ARG 52 Serine SER 100 Cysteine CYS 5 Leucine LEU 53 Tryptophan TRP 101 Glycine GLY 6 Leucine LEU 54 Histidine HIE 102 Proline PRO 7 Alanine ALA 55 Arginine ARG 103 Glycine GLY 8 Glutamic Acid GLU 56 Threonine THR 104 Tryptophan TRP 9 Leucine LEU 57 Alanine ALA 105 Valine VAL 10 Leucine LEU 58 Lysine LYS 106 Proline PRO 11 Asparagine ASN 59 Alanine ALA 107 Threonine THR 12 Alanine ALA 60 Arginine ARG 108 Proline PRO 13 Serine SER 61 Arginine ARG 109 Glycine GLY 14 Arginine ARG 62 Glycine GLY 110 Glycine GLY 15 Glycine GLY 63 Glycine GLY 111 Cysteine CYS 16 Glycine GLY 64 Arginine ARG 112 Leucine LEU 17 Aspartic Acid ASP 65 Cysteine CYS 113 Glycine GLY 18 Glycine GLY 66 Alanine ALA 114 Phenyalanine PHE 19 Serine SER 67 Alanine ALA 115 Phenyalanine PHE 20 Glycine GLY 68 Leucine LEU 116 Serine SER 21 Glutamic Acid GLU 69 Arginine ARG 117 Arginine ARG 22 Glycine GLY 70 Aspartic Acid ASP 118 Glutamic Acid GLU 23 Alanine ALA 71 Glutamic Acid GLU 119 Leucine LEU 24 Aspartic Acid ASP 72 Glutamic Acid GLU 120 Serine SER 25 Glycine GLY 73 Alanine ALA 121 Tryptophan TRP 26 Arginine ARG 74 Phenyalanine -

Impaired Amino Acid and TCA Metabolism and Cardiovascular Autonomic Neuropathy Progression in Type 1 Diabetes
Diabetes Volume 68, October 2019 2035 Impaired Amino Acid and TCA Metabolism and Cardiovascular Autonomic Neuropathy Progression in Type 1 Diabetes Anna V. Mathew,1 Mamta Jaiswal,2 Lynn Ang,2 George Michailidis,3 Subramaniam Pennathur,1,4 and Rodica Pop-Busui2 Diabetes 2019;68:2035–2044 | https://doi.org/10.2337/db19-0145 GENETICS/GENOMES/PROTEOMICS/METABOLOMICS While diabetes is characterized by hyperglycemia, nutri- Cardiovascular autonomic neuropathy (CAN) is a widely ent metabolic pathways like amino acid and tricarboxylic prevalent chronic diabetes complication that is character- acid (TCA) cycle are also profoundly perturbed. As gly- ized by impaired autonomic control of the cardiovascular cemic control alone does not prevent complications, we system (1). Although the initial prevalence of CAN in hypothesized that these metabolic disruptions are re- patients newly diagnosed with type 1 diabetes is low, later sponsible for the development and progression of di- prevalence after 15 years of diabetes increases to 35% in abetic cardiovascular autonomic neuropathy (CAN). We patients with type 1 diabetes and 60% in patients with performed standardized cardiovascular autonomic re- type 2 diabetes (1–4). CAN is an independent predictor of fl ex tests and targeted fasting plasma metabolomic chronic kidney disease progression and of cardiovascular analysis of amino acids and TCA cycle intermediates disease morbidity and mortality in patients with diabetes in subjects with type 1 diabetes and healthy control (5–8). CAN is also associated with an increased risk of subjects followed for 3 years. Forty-seven participants cardiac arrhythmias, silent myocardial ischemia, myocar- with type 1 diabetes (60% female and mean 6 SD age dial dysfunction, and sudden death (1,2,4,5,8). -

The Fate of Arginine and Proline Carbon in Squid Tissuesl
Pacific Science (1982), vol. 36, no. 3 © 1983 by the University of Hawaii Press. All rights reserved The Fate of Arginine and Proline Carbon in Squid Tissuesl T. P. MOMMSEN,2 C. J. FRENCH,2 B. EMMETI,2 and P. W. HOCHACHKA2 ABSTRACT: The metabolism of proline and arginine was investigated in kidney, gill, and heart of the pelagic squid, Symplectoteuthis. The rates of CO2 release from 14C-proline exceeded the rates from 14C-arginine. The metabolic rate of arginine and proline was assessed by monitoring the incorporation of arginine-derived carbon into various intermediates. Arginine was metabolized, through ornithine, to proline as well as to glutamate and various subsequent derivatives (alanine, octopine, aspartate, and carboxylic acids). The same com ponents became labeled using 14C-proline as the starting substrate, but only the gill was capable ofconverting proline to arginine via the urea cycle. In addition, 14C-proline oxidation rates were high enough to exceed those of 14C-glucose in at least three tissues, kidney, heart, and inner mantle muscle. AT LEAST IN PART because ofthe large pool size data for heart, gill, and kidney from the squid, of free amino acids in cephalopod muscles Symplectoteuthis, showing the capacity for ar (e.g., see Hochachka, French, and Meredith ginine conversion to proline. The conversion 1978), interest recently has been focusing ofproline to arginine was measurable only in on their possible roles in energy metabolism. the gill. Although qualitatively similar to re During metabolic studies on the 1979 Alpha sults obtained with other species, these data Helix Cephalopod Expedition, relatively high also show some important, tissue-specific dif rates of CO2 release from arginine and ferences (Mommsen et al. -

Arginine Is Synthesized from Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates
0031-3998/11/6901-0046 Vol. 69, No. 1, 2011 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2010 International Pediatric Research Foundation, Inc. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates CHRIS TOMLINSON, MAHROUKH RAFII, MICHAEL SGRO, RONALD O. BALL, AND PAUL PENCHARZ Department of Paediatrics [C.T., M.S., P.P.], Research Institute [C.T., M.R., P.P.], The Hospital for Sick Children, Toronto, Ontario M5G1X8, Canada; Department of Nutritional Sciences [C.T., M.S., P.P.], University of Toronto, Toronto, Ontario M5S3E2, Canada; Department of Paediatrics [M.S.], St Michael’s Hospital, Toronto, Ontario M5B1W8, Canada; Department of Agricultural, Food and Nutritional Science [R.O.B., P.P.], University of Alberta, Edmonton, Alberta T6G2P5, Canada ABSTRACT: In neonatal mammals, arginine is synthesized in the litis (NEC) (8) and pulmonary hypertension (9). Furthermore, enterocyte, with either proline or glutamate as the dietary precursor. arginine supplementation was shown to reduce the incidence We have shown several times in piglets that proline is the only of all stages of NEC in moderately at risk infants (10) and a precursor to arginine, although in vitro evidence supports glutamate single bolus infusion of i.v. arginine improved oxygenation in in this role. Because of this uncertainty, we performed a multitracer infants with pulmonary hypertension (11). Therefore, because stable isotope study to determine whether proline, glutamate, or both are dietary precursors for arginine in enterally fed human neonates. arginine is clearly important for metabolism in the neonate, it Labeled arginine (M ϩ 2), proline (M ϩ 1), and glutamate (M ϩ 3) is critical to understand the metabolic pathways involved in its were given enterally to 15 stable, growing preterm infants (GA at synthesis. -

Amino Acid Requirements of the Free-Living Nematode Caenorhabditis Briggsae
AMINO ACID REQUIREMENTS OF THE FREE-LIVING NEMATODE CAENORHABDITIS BRIGGSAE BY J. R. VANFLETEREN Instituut voor Dierkunde, Laboratoria voor Morfologie en Systematiek, RijksuniversiteitGent, Belgium Washed yeast ribosomes promote growth and reproduction of C. briggsae, even when supple- mented to the basal medium at dosages too low to provide the organisms with sufficient amounts of essential amino acids. Hence, a re-investigation of the amino acid requirements of C. briggsae by single and multiple omission of amino acids from the basal medium revealed unambiguously that arginine, histidine, lysine, tryptophan, phenylalanine, methionine, threonine, leucine, isoleucine and valine are not synthetized at levels to permit reproduction; they are called essential amino acids. The requirement for arginine and isoleucinehowever appears to be less clear-cut. On the contrary, evidence is presented that alanine, asparagine, cysteine, glutamate, glutamine, glycine, proline, serine and tryosine can be synthetized at adequate levels; they are called non- essential amino acids. In addition it was shown that multiple omission of the non-essential amino acids is not deleterious. This is believed to be an important step towards the development of a minimum essential medium (MEM) for growth and reproduction of C. briggsae. Sustained growth of the free-living nematode Caenorhabditis brigg.rae can be obtained on a chemically defined medium, supplemented with adequate levels of a proteinaceous growth factor. The most satisfactory, chemically defined medium hitherto reported (Buecher, Hansen & Yarwood, 1966), has been called C. brigg.iae Maintenance Medium (CbMM) and is now commercially available. CbMM is an extremely rich medium, being composed of 53 components, all present at high concentrations. -

Amino Acid Catabolism: Urea Cycle the Urea Bi-Cycle Two Issues
BI/CH 422/622 OUTLINE: OUTLINE: Protein Degradation (Catabolism) Digestion Amino-Acid Degradation Inside of cells Urea Cycle – dealing with the nitrogen Protein turnover Ubiquitin Feeding the Urea Cycle Activation-E1 Glucose-Alanine Cycle Conjugation-E2 Free Ammonia Ligation-E3 Proteosome Glutamine Amino-Acid Degradation Glutamate dehydrogenase Ammonia Overall energetics free Dealing with the carbon transamination-mechanism to know Seven Families Urea Cycle – dealing with the nitrogen 1. ADENQ 5 Steps 2. RPH Carbamoyl-phosphate synthetase oxidase Ornithine transcarbamylase one-carbon metabolism Arginino-succinate synthetase THF Arginino-succinase SAM Arginase 3. GSC Energetics PLP uses Urea Bi-cycle 4. MT – one carbon metabolism 5. FY – oxidases Amino Acid Catabolism: Urea Cycle The Urea Bi-Cycle Two issues: 1) What to do with the fumarate? 2) What are the sources of the free ammonia? a-ketoglutarate a-amino acid Aspartate transaminase transaminase a-keto acid Glutamate 1 Amino Acid Catabolism: Urea Cycle The Glucose-Alanine Cycle • Vigorously working muscles operate nearly anaerobically and rely on glycolysis for energy. a-Keto acids • Glycolysis yields pyruvate. – If not eliminated (converted to acetyl- CoA), lactic acid will build up. • If amino acids have become a fuel source, this lactate is converted back to pyruvate, then converted to alanine for transport into the liver. Excess Glutamate is Metabolized in the Mitochondria of Hepatocytes Amino Acid Catabolism: Urea Cycle Excess glutamine is processed in the intestines, kidneys, and liver. (deaminating) (N,Q,H,S,T,G,M,W) OAA à Asp Glutamine Synthetase This costs another ATP, bringing it closer to 5 (N,Q,H,S,T,G,M,W) 29 N 2 Amino Acid Catabolism: Urea Cycle Excess glutamine is processed in the intestines, kidneys, and liver. -

A Study of the Renal Tubular Absorption and Glycine and Histidine in Dogs Following the Intravenous Infusion of Concetrated Solutions of These Amino Acids Daniel M
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Yale Medicine Thesis Digital Library School of Medicine 1960 A study of the renal tubular absorption and glycine and histidine in dogs following the intravenous infusion of concetrated solutions of these amino acids Daniel M. Jones Yale University Follow this and additional works at: http://elischolar.library.yale.edu/ymtdl Recommended Citation Jones, Daniel M., "A study of the renal tubular absorption and glycine and histidine in dogs following the intravenous infusion of concetrated solutions of these amino acids" (1960). Yale Medicine Thesis Digital Library. 2755. http://elischolar.library.yale.edu/ymtdl/2755 This Open Access Thesis is brought to you for free and open access by the School of Medicine at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Yale Medicine Thesis Digital Library by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. YALE MEDICAL LIBRARY Manuscript Theses Unpublished theses submitted for the Master's and Doctor's degrees and deposited in the Yale Medical Library are to be used only with due regard to the rights of the authors. Bibliographical references may be noted, but passages must not be copied without permission of the authors, and without proper credit being given in subsequent written or published work. This thesis by has been used by the following persons, whose signatures attest their acceptance of the above restrictions. NAME AND ADDRESS DATE Digitized by the Internet Archive in 2017 with funding from The National Endowment for the Humanities and the Arcadia Fund https://archive.org/details/studyofrenaltubuOOjone A STUDY OF THE RENAL TUBULAR ABSORPTION AND EXCRETION OF GLYCINE AND HISTIDINE IN DOGS FOLLOWING THE INTRAVENOUS INFUSION OF CONCENTRATED SOLUTIONS OF THESE AMINO ACIDS DANIEL M. -

Substitution of a Single Amino Acid (Aspartic Acid for Histidine)
Proc. Nail. Acad. Sci. USA Vol. 87, pp. 6868-6872, September 1990 Immunology Substitution of a single amino acid (aspartic acid for histidine) converts the functional activity of human complement C4B to C4A (thioester/immune complexes/C4 polymorphism) MICHAEL C. CARROLL*t, DEHMANHI M. FATHALLAH*t, LUIGI BERGAMASCHINI*§, ELIZABETH M. ALICOT*, AND DAVID E. ISENMAN¶ *Department of Pathology, Harvard Medical School and Children's Hospital, Boston, MA 02115; and $1Department of Biochemistry, University of Toronto, Toronto, ON, M5S 1A8 Canada Communicated by Elkan Blout, June 15, 1990 ABSTRACT The C4B isotype of the fourth component of ative or simply serves as a marker for a linked disease human complement (C4) displays 3- to 4-fold greater hemolytic susceptibility gene is not known. Nevertheless, C4 polymor- activity than does its other isotype C4A. This correlates with phism in general, and coexpression of the C4A and C4B differences in their covalent binding efficiencies to erythrocytes isotypes in particular, may ensure interaction with a wide coated with antibody and complement C1. C4A binds to a range of surfaces, similar to what has been proposed for class greater extent when C1 is on IgG immune aggregates. The I and II major histocompatibility molecules (19). differences in covalent binding properties correlate only with C4 protein is synthesized as a single polypeptide of 1706 amino acid changes between residues 1101 and 1106 (pro-C4 amino acids (13), which is processed (20) into three subunits numbering)-namely, Pro-1101, Cys-1102, Leu-1105, and (a, 95 kDa; ,3, 75 kDa; and y, 30 kDa) (21) before secretion.