The Fate of Arginine and Proline Carbon in Squid Tissuesl
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CCA One Care Options Formulary
Commonwealth Care Alliance One Care Plan (Medicare-Medicaid Plan) 2021 List of Covered Drugs (Formulary) 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN For more recent information or other questions, contact Commonwealth Care Alliance Member Services at 1-866-610-2273 (TTY: call MassRelay at 711), 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthonecare.org H0137_CF2021 Approved Formulary: ID 00021588 • Version 13 • Updated on 08/01/2021 One Care Plan | 2021 List of Covered Drugs (Formulary) Introduction This document is called the List of Covered Drugs (also known as the Drug List). It tells you which prescription drugs, over-the-counter drugs and items are covered by Commonwealth Care Alliance. The Drug List also tells you if there are any special rules or restrictions on any drugs covered by One Care. Key terms and their definitions appear in the last chapter of the Member Handbook. Table of Contents A. Disclaimers ........................................................................................................................ 4 B. Frequently Asked Questions (FAQ) .................................................................................. 5 What prescription drugs are on the List of Covered Drugs? (We call the List of Covered Drugs the “Drug List” for short.) ................................................................... 5 B2. Does the Drug List ever change? ............................................................................... 5 B3. What happens when there is a change to the Drug List? ........................................... 6 B4. Are there any restrictions or limits on drug coverage or any required actions to take to get certain drugs? .................................................................................................. 7 B5. How will you know if the drug you want has limitations or if there are required actions to take to get the drug? ................................................................................. -

Al Exposure Increases Proline Levels by Different Pathways in An
www.nature.com/scientificreports OPEN Al exposure increases proline levels by diferent pathways in an Al‑sensitive and an Al‑tolerant rye genotype Alexandra de Sousa1,2, Hamada AbdElgawad2,4, Fernanda Fidalgo1, Jorge Teixeira1, Manuela Matos3, Badreldin A. Hamed4, Samy Selim5, Wael N. Hozzein6, Gerrit T. S. Beemster2 & Han Asard2* Aluminium (Al) toxicity limits crop productivity, particularly at low soil pH. Proline (Pro) plays a role in protecting plants against various abiotic stresses. Using the relatively Al‑tolerant cereal rye (Secale cereale L.), we evaluated Pro metabolism in roots and shoots of two genotypes difering in Al tolerance, var. RioDeva (sensitive) and var. Beira (tolerant). Most enzyme activities and metabolites of Pro biosynthesis were analysed. Al induced increases in Pro levels in each genotype, but the mechanisms were diferent and were also diferent between roots and shoots. The Al‑tolerant genotype accumulated highest Pro levels and this stronger increase was ascribed to simultaneous activation of the ornithine (Orn)‑biosynthetic pathway and decrease in Pro oxidation. The Orn pathway was particularly enhanced in roots. Nitrate reductase (NR) activity, N levels, and N/C ratios demonstrate that N‑metabolism is less inhibited in the Al‑tolerant line. The correlation between Pro changes and diferences in Al‑sensitivity between these two genotypes, supports a role for Pro in Al tolerance. Our results suggest that diferential responses in Pro biosynthesis may be linked to N‑availability. Understanding the role of Pro in diferences between genotypes in stress responses, could be valuable in plant selection and breeding for Al resistance. Proline (Pro) is involved in a wide range of plant physiological and developmental processes1. -

Amino Acid Recognition by Aminoacyl-Trna Synthetases
www.nature.com/scientificreports OPEN The structural basis of the genetic code: amino acid recognition by aminoacyl‑tRNA synthetases Florian Kaiser1,2,4*, Sarah Krautwurst3,4, Sebastian Salentin1, V. Joachim Haupt1,2, Christoph Leberecht3, Sebastian Bittrich3, Dirk Labudde3 & Michael Schroeder1 Storage and directed transfer of information is the key requirement for the development of life. Yet any information stored on our genes is useless without its correct interpretation. The genetic code defnes the rule set to decode this information. Aminoacyl-tRNA synthetases are at the heart of this process. We extensively characterize how these enzymes distinguish all natural amino acids based on the computational analysis of crystallographic structure data. The results of this meta-analysis show that the correct read-out of genetic information is a delicate interplay between the composition of the binding site, non-covalent interactions, error correction mechanisms, and steric efects. One of the most profound open questions in biology is how the genetic code was established. While proteins are encoded by nucleic acid blueprints, decoding this information in turn requires proteins. Te emergence of this self-referencing system poses a chicken-or-egg dilemma and its origin is still heavily debated 1,2. Aminoacyl-tRNA synthetases (aaRSs) implement the correct assignment of amino acids to their codons and are thus inherently connected to the emergence of genetic coding. Tese enzymes link tRNA molecules with their amino acid cargo and are consequently vital for protein biosynthesis. Beside the correct recognition of tRNA features3, highly specifc non-covalent interactions in the binding sites of aaRSs are required to correctly detect the designated amino acid4–7 and to prevent errors in biosynthesis5,8. -

Low Proline Diet in Type I Hyperprolinaemia
Arch Dis Child: first published as 10.1136/adc.46.245.72 on 1 February 1971. Downloaded from Archives of Disease in Childhood, 1971, 46, 72. Low Proline Diet in Type I Hyperprolinaemia J. T. HARRIES, A. T. PIESOWICZ,* J. W. T. SEAKINS, D. E. M. FRANCIS, and 0. H. WOLFF From The Hospital for Sick Children, and the Institute of Child Health, University of London Harries, J. T., Piesowicz, A. T., Seakins, J. W. T., Francis, D. E. M., and Wolff, 0. H. (1971). Archives of Disease in Childhood, 46, 72. Low proline diet in type I hyperprolinaemia. A diagnosis of Type I hyperprolinaemia was made in a 7-month-old infant who presented with hypocalcaemic convulsions and malabsorp- tion. The plasma levels of proline were grossly raised and the urinary excretion of proline, hydroxyproline, and glycine was increased; neurological development was delayed and there were associated abnormalities of the electroencephalogram, renal tract, and bones. Restriction of dietary proline at the age of 9 months resulted in a prompt fall of plasma levels of proline to normal, and a low proline diet was continued until the age of 27 months when persistence of the biochemical defect was shown. During the period of dietary treatment, growth was satisfactory, mental development improved, and the electroencephalogram, and the renal, skeletal, and intestinal abnormalities disappeared. Proline should be regarded as a 'semi-essential' amino acid in the growing infant. Hyperprolinaemia, appearing in several members balance can be maintained on a proline-free diet copyright. of a family was first described by Scriver, Schafer, (Rose et al., 1955), and therefore the amino acid is and Efron in 1961. -

Amino Acids Amino Acids
Amino Acids Amino Acids What Are Amino Acids? Essential Amino Acids Non Essential Amino Acids Amino acids are the building blocks of proteins; proteins are made of amino acids. Isoleucine Arginine (conditional) When you ingest a protein your body breaks it down into the individual aminos, Leucine Glutamine (conditional) reorders them, re-folds them, and turns them into whatever is needed by the body at Lysine Tyrosine (conditional) that time. From only 20 amino acids, the body is able to make thousands of unique proteins with different functions. Methionine Cysteine (conditional) Phenylalanine Glycine (conditional) Threonine Proline (conditional) Did You Know? Tryptophan Serine (conditional) Valine Ornithine (conditional) There are 20 different types of amino acids that can be combined to make a protein. Each protein consists of 50 to 2,000 amino acids that are connected together in a specific Histidine* Alanine sequence. The sequence of the amino acids determines each protein’s unique structure Asparagine and its specific function in the body. Asparate Popular Amino Acid Supplements How Do They Benefit Our Health? Acetyl L- Carnitine: As part of its role in supporting L-Lysine: L-Lysine, an essential amino acid, is mental function, Acetyl L-Carnitine may help needed to support proper growth and bone Proteins (amino acids) are needed by your body to maintain muscles, bones, blood, as support memory, attention span and mental development. It can also support immune function. well as create enzymes, neurotransmitters and antibodies, as well as transport and performance. store molecules. N-Acetyl Cysteine: N-Acetyl Cysteine (NAC) is a L-Arginine: L-Arginine is a nonessential amino acid form of the amino acid cysteine. -

Proline Oxidase Controls Proline, Glutamate, and Glutamine Cellular Concentrations in a U87 Glioblastoma Cell Line
RESEARCH ARTICLE Proline oxidase controls proline, glutamate, and glutamine cellular concentrations in a U87 glioblastoma cell line Pamela Cappelletti1,2*, Elena Tallarita1, Valentina Rabattoni1, Paola Campomenosi1,2, Silvia Sacchi1,2, Loredano Pollegioni1,2 1 Department of Biotechnology and Life Sciences, University of Insubria, Varese, Italy, 2 The Protein Factory Research Center, Politecnico of Milano and University of Insubria, Milano, Italy a1111111111 * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract L-Proline is a multifunctional amino acid that plays an essential role in primary metabolism and physiological functions. Proline is oxidized to glutamate in the mitochondria and the FAD-containing enzyme proline oxidase (PO) catalyzes the first step in L-proline degrada- OPEN ACCESS tion pathway. Alterations in proline metabolism have been described in various human dis- Citation: Cappelletti P, Tallarita E, Rabattoni V, eases, such as hyperprolinemia type I, velo-cardio-facial syndrome/Di George syndrome, Campomenosi P, Sacchi S, Pollegioni L (2018) schizophrenia and cancer. In particular, the mutation giving rise to the substitution Leu441- Proline oxidase controls proline, glutamate, and glutamine cellular concentrations in a U87 Pro was identified in patients suffering of schizophrenia and hyperprolinemia type I. Here, glioblastoma cell line. PLoS ONE 13(4): e0196283. we report on the expression of wild-type and L441P variants of human PO in a U87 glioblas- https://doi.org/10.1371/journal.pone.0196283 toma human cell line in an attempt to assess their effect on glutamate metabolism. The sub- Editor: Suzie Chen, Rutgers University, UNITED cellular localization of the flavoenzyme is not altered in the L441P variant, for which specific STATES activity is halved compared to the wild-type PO. -

List of Toxic Chemicals Within the Glycol Ethers Category
United States Office of Environmental Revised December 2000 Environmental Protection Information EPA 745-R-00-004 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY List of Toxic Chemicals within the Glycol Ethers Category Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. CONTENTS Section 1. Introduction ...................................................... 3 Section 2. CAS Number List of Some Chemicals within the Glycol Ethers Category ........ 6 Section 3. CAS Number List of Some Mixtures That Contain Glycol Ethers within the Category .............................................. 185 Section 4. CAS Number List of Some Oligomeric or Polymeric Chemicals That Might Contain Glycol Ether Components within the Category .......................... 187 FOREWORD This document is an updated version of the previous document, EPA 745-R-99-006, June 1999. This version has the following updates: • The titles to Table 1 on page 6, Table 2 on page 185, and Table 3 on 187 are modified; and • The CAS number of second listing in Table 3 (Poly(oxy-1,2-ethanediyl), .alpha.- (phenylsulfonyl)-.omega.-methoxy-) on page 187 is changed from 7664-41-7 to 67584-43-4. -
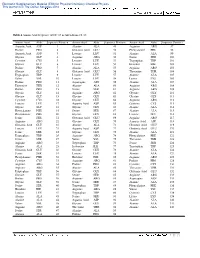
Table 2 Amino Acid Sequence of OC-17 As Taken from Ref. 28 Amino
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics This journal is © The Owner Societies 2012 Table 2 Amino Acid Sequence of OC-17 as taken from ref. 28 Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Aspartic Acid ASP 1 Alanine ALA 49 Arginine ARG 97 Proline PRO 2 Glutamic Acid GLU 50 Phenyalanine PHE 98 Aspartic Acid ASP 3 Leucine LEU 51 Alanine ALA 99 Glycine GLY 4 Arginine ARG 52 Serine SER 100 Cysteine CYS 5 Leucine LEU 53 Tryptophan TRP 101 Glycine GLY 6 Leucine LEU 54 Histidine HIE 102 Proline PRO 7 Alanine ALA 55 Arginine ARG 103 Glycine GLY 8 Glutamic Acid GLU 56 Threonine THR 104 Tryptophan TRP 9 Leucine LEU 57 Alanine ALA 105 Valine VAL 10 Leucine LEU 58 Lysine LYS 106 Proline PRO 11 Asparagine ASN 59 Alanine ALA 107 Threonine THR 12 Alanine ALA 60 Arginine ARG 108 Proline PRO 13 Serine SER 61 Arginine ARG 109 Glycine GLY 14 Arginine ARG 62 Glycine GLY 110 Glycine GLY 15 Glycine GLY 63 Glycine GLY 111 Cysteine CYS 16 Glycine GLY 64 Arginine ARG 112 Leucine LEU 17 Aspartic Acid ASP 65 Cysteine CYS 113 Glycine GLY 18 Glycine GLY 66 Alanine ALA 114 Phenyalanine PHE 19 Serine SER 67 Alanine ALA 115 Phenyalanine PHE 20 Glycine GLY 68 Leucine LEU 116 Serine SER 21 Glutamic Acid GLU 69 Arginine ARG 117 Arginine ARG 22 Glycine GLY 70 Aspartic Acid ASP 118 Glutamic Acid GLU 23 Alanine ALA 71 Glutamic Acid GLU 119 Leucine LEU 24 Aspartic Acid ASP 72 Glutamic Acid GLU 120 Serine SER 25 Glycine GLY 73 Alanine ALA 121 Tryptophan TRP 26 Arginine ARG 74 Phenyalanine -

Arginine Is Synthesized from Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates
0031-3998/11/6901-0046 Vol. 69, No. 1, 2011 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2010 International Pediatric Research Foundation, Inc. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates CHRIS TOMLINSON, MAHROUKH RAFII, MICHAEL SGRO, RONALD O. BALL, AND PAUL PENCHARZ Department of Paediatrics [C.T., M.S., P.P.], Research Institute [C.T., M.R., P.P.], The Hospital for Sick Children, Toronto, Ontario M5G1X8, Canada; Department of Nutritional Sciences [C.T., M.S., P.P.], University of Toronto, Toronto, Ontario M5S3E2, Canada; Department of Paediatrics [M.S.], St Michael’s Hospital, Toronto, Ontario M5B1W8, Canada; Department of Agricultural, Food and Nutritional Science [R.O.B., P.P.], University of Alberta, Edmonton, Alberta T6G2P5, Canada ABSTRACT: In neonatal mammals, arginine is synthesized in the litis (NEC) (8) and pulmonary hypertension (9). Furthermore, enterocyte, with either proline or glutamate as the dietary precursor. arginine supplementation was shown to reduce the incidence We have shown several times in piglets that proline is the only of all stages of NEC in moderately at risk infants (10) and a precursor to arginine, although in vitro evidence supports glutamate single bolus infusion of i.v. arginine improved oxygenation in in this role. Because of this uncertainty, we performed a multitracer infants with pulmonary hypertension (11). Therefore, because stable isotope study to determine whether proline, glutamate, or both are dietary precursors for arginine in enterally fed human neonates. arginine is clearly important for metabolism in the neonate, it Labeled arginine (M ϩ 2), proline (M ϩ 1), and glutamate (M ϩ 3) is critical to understand the metabolic pathways involved in its were given enterally to 15 stable, growing preterm infants (GA at synthesis. -

Amino Acid Catabolism: Urea Cycle the Urea Bi-Cycle Two Issues
BI/CH 422/622 OUTLINE: OUTLINE: Protein Degradation (Catabolism) Digestion Amino-Acid Degradation Inside of cells Urea Cycle – dealing with the nitrogen Protein turnover Ubiquitin Feeding the Urea Cycle Activation-E1 Glucose-Alanine Cycle Conjugation-E2 Free Ammonia Ligation-E3 Proteosome Glutamine Amino-Acid Degradation Glutamate dehydrogenase Ammonia Overall energetics free Dealing with the carbon transamination-mechanism to know Seven Families Urea Cycle – dealing with the nitrogen 1. ADENQ 5 Steps 2. RPH Carbamoyl-phosphate synthetase oxidase Ornithine transcarbamylase one-carbon metabolism Arginino-succinate synthetase THF Arginino-succinase SAM Arginase 3. GSC Energetics PLP uses Urea Bi-cycle 4. MT – one carbon metabolism 5. FY – oxidases Amino Acid Catabolism: Urea Cycle The Urea Bi-Cycle Two issues: 1) What to do with the fumarate? 2) What are the sources of the free ammonia? a-ketoglutarate a-amino acid Aspartate transaminase transaminase a-keto acid Glutamate 1 Amino Acid Catabolism: Urea Cycle The Glucose-Alanine Cycle • Vigorously working muscles operate nearly anaerobically and rely on glycolysis for energy. a-Keto acids • Glycolysis yields pyruvate. – If not eliminated (converted to acetyl- CoA), lactic acid will build up. • If amino acids have become a fuel source, this lactate is converted back to pyruvate, then converted to alanine for transport into the liver. Excess Glutamate is Metabolized in the Mitochondria of Hepatocytes Amino Acid Catabolism: Urea Cycle Excess glutamine is processed in the intestines, kidneys, and liver. (deaminating) (N,Q,H,S,T,G,M,W) OAA à Asp Glutamine Synthetase This costs another ATP, bringing it closer to 5 (N,Q,H,S,T,G,M,W) 29 N 2 Amino Acid Catabolism: Urea Cycle Excess glutamine is processed in the intestines, kidneys, and liver. -

Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat
Pediat. Res. 6: 713-719 (1972) Amino acid neonate intestine transport, amino acid Amino Acid Transport Pathways in the Small Intestine of the Neonatal Rat J. F. FITZGERALD1431, S. REISER, AND P. A. CHRISTIANSEN Departments of Pediatrics, Medicine, and Biochemistry, and Gastrointestinal Research Laboratory, Indiana University School of Medicine and Veterans Administration Hospital, Indianapolis, Indiana, USA Extract The activity of amino acid transport pathways in the small intestine of the 2-day-old rat was investigated. Transport was determined by measuring the uptake of 1 mM con- centrations of various amino acids by intestinal segments after a 5- or 10-min incuba- tion and it was expressed as intracellular accumulation. The neutral amino acid transport pathway was well developed with intracellular accumulation values for leucine, isoleucine, valine, methionine, tryptophan, phenyl- alanine, tyrosine, and alanine ranging from 3.9-5.6 mM/5 min. The intracellular accumulation of the hydroxy-containing neutral amino acids threonine (essential) and serine (nonessential) were 2.7 mM/5 min, a value significantly lower than those of the other neutral amino acids. The accumulation of histidine was also well below the level for the other neutral amino acids (1.9 mM/5 min). The basic amino acid transport pathway was also operational with accumulation values for lysine, arginine and ornithine ranging from 1.7-2.0 mM/5 min. Accumulation of the essential amino acid lysine was not statistically different from that of nonessential ornithine. Ac- cumulation of aspartic and glutamic acid was only 0.24-0.28 mM/5 min indicating a very low activity of the acidic amino acid transport pathway. -

Increased Arginine Amino Aciduria/Urea Cycle Disorder
Newborn Screening ACT Sheet Increased Arginine Amino Aciduria/Urea Cycle Disorder Differential Diagnosis: Argininemia (ARG) Condition Description: The urea cycle is the enzyme cycle whereby ammonia is converted to urea. In argininemia, defects in arginase, a urea cycle enzyme, may result in hyperammonemia. Take the Following IMMEDIATE Actions • Contact family to inform them of the newborn screening result and ascertain clinical status (poor feeding, vomiting, lethargy, tachypnea). • Immediate telephone consultation with pediatric metabolic specialist. (See attached list.) • Evaluate the newborn (poor feeding, vomiting, lethargy, hypotonia, tachypnea, seizures and signs of liver disease). • If any sign is present or infant is ill, IMMEDIATELY initiate emergency treatment for hyperammonemia in consultation with metabolic specialist. • Transport to hospital for further treatment in consultation with metabolic specialist. • Initiate timely confirmatory/diagnostic testing and management, as recommended by specialist. • Initial testing: immediate plasma ammonia, plasma quantitative amino acids, and urine orotic acid. • Repeat newborn screen if second screen has not been done. • Provide family with basic information about hyperammonemia. • Report findings to newborn screening program. Diagnostic Evaluation: Specific diagnosis is made by plasma quantitative amino acid analysis revealing increased arginine and urine orotic acid analysis revealing increased orotic acid, respectively. Blood ammonia determination may also reveal hyperammonemia. Clinical