List of Toxic Chemicals Within the Glycol Ethers Category
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CCA One Care Options Formulary
Commonwealth Care Alliance One Care Plan (Medicare-Medicaid Plan) 2021 List of Covered Drugs (Formulary) 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN For more recent information or other questions, contact Commonwealth Care Alliance Member Services at 1-866-610-2273 (TTY: call MassRelay at 711), 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthonecare.org H0137_CF2021 Approved Formulary: ID 00021588 • Version 13 • Updated on 08/01/2021 One Care Plan | 2021 List of Covered Drugs (Formulary) Introduction This document is called the List of Covered Drugs (also known as the Drug List). It tells you which prescription drugs, over-the-counter drugs and items are covered by Commonwealth Care Alliance. The Drug List also tells you if there are any special rules or restrictions on any drugs covered by One Care. Key terms and their definitions appear in the last chapter of the Member Handbook. Table of Contents A. Disclaimers ........................................................................................................................ 4 B. Frequently Asked Questions (FAQ) .................................................................................. 5 What prescription drugs are on the List of Covered Drugs? (We call the List of Covered Drugs the “Drug List” for short.) ................................................................... 5 B2. Does the Drug List ever change? ............................................................................... 5 B3. What happens when there is a change to the Drug List? ........................................... 6 B4. Are there any restrictions or limits on drug coverage or any required actions to take to get certain drugs? .................................................................................................. 7 B5. How will you know if the drug you want has limitations or if there are required actions to take to get the drug? ................................................................................. -

Of Grignard Reagent Formation. the Surface Nature of the Reaction
286 Ace. Chem. Res. 1990,23, 286-293 Mechanism of Grignard Reagent Formation. The Surface Nature of the Reaction H. M. WALBORSKY Dittmer Laboratory of Chemistry, Florida State University, Tallahassee, Florida 32306 Received February 23, 1990 (Revised Manuscript Received May 7, 1990) The reaction of organic halides (Br, C1, I) with mag- Scheme I nesium metal to yield what is referred to today as a Kharasch-Reinmuth Mechanism for Grignard Reagent Grignard reagent has been known since the turn of the Formation century,' The name derives from its discoverer, Nobel (1)(Mg0)AMg*)2y + RX 4 [(M~'~(MQ')~~-,('MQX)+ R.] + laureate Victor Grignard. How this reagent is formed, (Mgo)x-2(MQ')2~MgX)(MgR) that is, how a magnesium atom is inserted into a car- bon-halogen bond, is the subject of this Account. ('4 (Ms0),-*(M9')2~MgX)(MgR) + + (Mg0)x-dMg*)2y+2 + 2RMgX RX + Mg - RMgX Kharasch and Reinmuth,, persuaded by the work of late under the same conditions gave Itl = 6.2 X s-l. Another system that meets the above criterion is the Gomberg and Bachmad as well as by product analyses of many Grignard formation reactions that existed in vinyl system. The lack of reactivity of vinyl halides toward SN1reactions is well-known and is exemplified the literature prior to 1954,speculated that the reaction involved radicals and that the radical reactions might by the low solvolysis rate of 2-propenyl triflate5 in 80% involve "surface adherent radicals, at least in part". The ethanol at 25 OC, kl being 9.8 X s-l. -

Methoxyethane, Physical Properties, Safety, MSDS, Enthalpy, Gas Liquid
(c) Bürkle GmbH 2021 Important Important information The tables “Chemical resistance of plastics”, “Plastics and their properties” and “Viscosity of liquids" as well as the information about chemical resistance given in the particular product descriptions have been drawn up based on information provided by various raw material manufacturers. These values are based solely on laboratory tests with raw materials. Plastic components produced from these raw materials are frequently subject to influences that cannot be recognized in laboratory tests (temperature, pressure, material stress, effects of chemicals, construction features, etc.). For this reason the values given are only to be regarded as being guidelines. In critical cases it is essential that a test is carried out first. No legal claims can be derived from this information; nor do we accept any liability for it. A knowledge of the chemical and mechanical resistance alone is not sufficient for the evaluation of the usability of a product. For example, the regulations concerning flammable liquids (explosion prevention) must also be taken into consideration. Copyright This table has been published and updated by Bürkle GmbH, D-79415 Bad Bellingen as a work of reference. This Copyright clause must not be removed. The table may be freely passed on and copied, provided that the information about the publisher is retained. Extensions, additions and translations If your own experiences with materials and media could be used to extend this table then we would be pleased to receive any additional information. Please send an E-Mail to [email protected]. We would also like to receive translations into other languages. -

United States Patent (19) 11) Patent Number: 4,585,788 Helsley Et Al
United States Patent (19) 11) Patent Number: 4,585,788 Helsley et al. 45) Date of Patent: Apr. 29, 1986 54 6,11-DIHYDRODIBENZB,EOXEPIN ACETIC ACIDS AND DERVATIVES FOREIGN PATENT DOCUMENTS 48-389 6/1972 Japan . 75 Inventors: Grover C. Helsley, Pottersville; 000425 7/1972 Japan. Arthur R. McFadden, East Brunswick, both of N.J.; David Primary Examiner-Norma S. Milestone Hoffman, North Kingstown, R.I. Attorney, Agent, or Firm-Curtis, Morris & Safford 57 ABSTRACT (73) Assignee: American Hoechst Corporation, 6,11-Dihydrodibenzb,eoxepin-acetic acids and deriva Somerville, N.J. tives having the general formula 21 Appl. No.: 459,774 1 22 Fied: Apr. 10, 1974 210 X Yn C-Z Related U.S. Application Data St. 5 k 63 Continuation-in-part of Ser. No. 394,801, Sep. 6, 1973, abandoned. (51) Int. Cl." .................. C07D 313/12; A61K 31/335 are prepared by multi-step sequences. X is C=O, CHCl, 52) U.S. C. ..................................... 514/450; 549/354 CHBr, CH2 or CHOR; Y is alkyl or alkoxy of 1 to 4 (58) Field of Search ........................ 260/333; 549/354; carbon atoms, halogen or trifluoromethyl; n is 0, 1, 2 or 514/450 3; Z is COOR5, CH2OR5, CONR25 or CONHOR5; and Rl-Rs are hydrogen or alkyl of 1 to 4 carbon atoms. 56) References Cited These compounds and the physiologically tolerable U.S. PATENT DOCUMENTS salts thereof are useful as antiinflammatory and analge 3,702,852 11/1972 Yale et al. ........................... 260/333 sic agents. 3,714,201 1/1973 Yale et al. ........................... 260/333 3,758,528 9/1973 Malen et al. -

Triethylene Glycol
Safety Data Sheet Triethylene Glycol 1. PRODUCT AND COMPANY IDENTIFICATION Product Name: Triethylene Glycol Synonyms/Generic Names: Triglycol; 2,2'-[1,2-Ethanediylbis(oxy)]bisethanol Product Number: 5965 Product Use: Industrial, Manufacturing or Laboratory use Manufacturer: Columbus Chemical Industries, Inc. N4335 Temkin Rd. Columbus, WI. 53925 For More Information: 920-623-2140 (Monday-Friday 8:00-4:30) www.columbuschemical.com In Case of Emergency Call: CHEMTREC - 800-424-9300 or 703-527-3887 (24 Hours/Day, 7 Days/Week) 2. HAZARDS IDENTIFICATION OSHA Hazards: No known OSHA hazards Target Organs: None Signal Word: Warning Pictograms: GHS Classification: Skin irritation Category 3 Eye irritation Category 2B GHS Label Elements, including precautionary statements: Hazard Statements: H316 Causes mild skin irritation. H320 Causes eye irritation. Precautionary Statements: P264 Wash hands thoroughly after handling. P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P332+P313 If skin irritation occurs: Get medical advice/attention. P337+P313 If eye irritation persists: Get medical advice/attention. Revised on 09/27/2018 Page 1 of 6 Potential Health Effects Eyes May cause eye irritation. Inhalation May be harmful if inhaled. Causes respiratory tract irritation. Skin May be harmful if absorbed through skin. Causes skin irritation. Ingestion May be harmful if swallowed. NFPA Ratings HMIS Ratings Health 1 Health 1 Flammability 0 Fire 0 Reactivity 0 Reactivity 0 Specific hazard Not Available Personal Not Available 3. COMPOSITION/INFORMATION ON INGREDIENTS EINECS# / Molecular Component Weight % CAS # Formula ELINCS# Weight Triethylene Glycol >97 112-27-6 203-953-2 C6H14O4 150.17 g/mol Diethylene Glycol <3 111-46-6 203-872-2 C4H10O3 106.12 g/mol 4. -
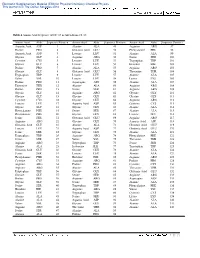
Table 2 Amino Acid Sequence of OC-17 As Taken from Ref. 28 Amino
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics This journal is © The Owner Societies 2012 Table 2 Amino Acid Sequence of OC-17 as taken from ref. 28 Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Aspartic Acid ASP 1 Alanine ALA 49 Arginine ARG 97 Proline PRO 2 Glutamic Acid GLU 50 Phenyalanine PHE 98 Aspartic Acid ASP 3 Leucine LEU 51 Alanine ALA 99 Glycine GLY 4 Arginine ARG 52 Serine SER 100 Cysteine CYS 5 Leucine LEU 53 Tryptophan TRP 101 Glycine GLY 6 Leucine LEU 54 Histidine HIE 102 Proline PRO 7 Alanine ALA 55 Arginine ARG 103 Glycine GLY 8 Glutamic Acid GLU 56 Threonine THR 104 Tryptophan TRP 9 Leucine LEU 57 Alanine ALA 105 Valine VAL 10 Leucine LEU 58 Lysine LYS 106 Proline PRO 11 Asparagine ASN 59 Alanine ALA 107 Threonine THR 12 Alanine ALA 60 Arginine ARG 108 Proline PRO 13 Serine SER 61 Arginine ARG 109 Glycine GLY 14 Arginine ARG 62 Glycine GLY 110 Glycine GLY 15 Glycine GLY 63 Glycine GLY 111 Cysteine CYS 16 Glycine GLY 64 Arginine ARG 112 Leucine LEU 17 Aspartic Acid ASP 65 Cysteine CYS 113 Glycine GLY 18 Glycine GLY 66 Alanine ALA 114 Phenyalanine PHE 19 Serine SER 67 Alanine ALA 115 Phenyalanine PHE 20 Glycine GLY 68 Leucine LEU 116 Serine SER 21 Glutamic Acid GLU 69 Arginine ARG 117 Arginine ARG 22 Glycine GLY 70 Aspartic Acid ASP 118 Glutamic Acid GLU 23 Alanine ALA 71 Glutamic Acid GLU 119 Leucine LEU 24 Aspartic Acid ASP 72 Glutamic Acid GLU 120 Serine SER 25 Glycine GLY 73 Alanine ALA 121 Tryptophan TRP 26 Arginine ARG 74 Phenyalanine -

Revised Group Additivity Values for Enthalpies of Formation (At 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds
Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon– Hydrogen and Carbon–Hydrogen–Oxygen Compounds Cite as: Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 Submitted: 17 January 1996 . Published Online: 15 October 2009 N. Cohen ARTICLES YOU MAY BE INTERESTED IN Additivity Rules for the Estimation of Molecular Properties. Thermodynamic Properties The Journal of Chemical Physics 29, 546 (1958); https://doi.org/10.1063/1.1744539 Critical Evaluation of Thermochemical Properties of C1–C4 Species: Updated Group- Contributions to Estimate Thermochemical Properties Journal of Physical and Chemical Reference Data 44, 013101 (2015); https:// doi.org/10.1063/1.4902535 Estimation of the Thermodynamic Properties of Hydrocarbons at 298.15 K Journal of Physical and Chemical Reference Data 17, 1637 (1988); https:// doi.org/10.1063/1.555814 Journal of Physical and Chemical Reference Data 25, 1411 (1996); https://doi.org/10.1063/1.555988 25, 1411 © 1996 American Institute of Physics for the National Institute of Standards and Technology. Revised Group Additivity Values for Enthalpies of Formation (at 298 K) of Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds N. Cohen Thermochemical Kinetics Research, 6507 SE 31st Avenue, Portland, Oregon 97202-8627 Received January 17, 1996; revised manuscript received September 4, 1996 A program has been undertaken for the evaluation and revision of group additivity values (GAVs) necessary for predicting, by means of Benson's group additivity method, thermochemical properties of organic molecules. This review reports on the portion of that program dealing with GAVs for enthalpies of formation at 298.15 K (hereinafter abbreviated as 298 K) for carbon-hydrogen and carbon-hydrogen-oxygen compounds. -

(ESI) for Chemical Science. This Journal Is © the Royal Society of Chemistry 2015
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2015 Angelina Cayuelaa, Stuart R. Kennedyb, M. Laura Sorianoa, Miguel Valcárcel*a and Jonathan W. Steed*b Supplementary information -1 Figure S1. Fluorescence emission spectra of aqueous CD solutions (1 mg mL , 휆ex = 365 nm for c-CD and 휆ex = 370 nm for the others). -1 Figure S2. Fluorescence emission spectra of aqueous CD solutions (1 mg mL , ex = 365 nm for c-CD and 휆ex = 370 nm for the others) containing 1 w.t. % CD-gels with 1a at the same CD concentration and 2% 1-butyl-3-methylimidazolium tetrafluoroborate (BMIM-BF4). Figure S3. Photoluminescent response to 10 µg mL-1 of metal ions to a-CD solutions in the presence -1 of dissolved gelator (1 mg mL ) and a-CD-containing hydrogels (a-CDGs) at ex = 370 nm and em = 445 nm. Figure S4. SEM micrograph of the chromium-coated xerogel of 1a 1 wt%. Figure S5 hydrogel of gelator 1a at 2 wt% after standing for 1 day. Figure S6 a-, c-, p- and t-CD gels under ambient lighting, corresponding to Figure 6a in the main paper. Gelation and solubility studies carried out on compounds 1a, 1b, 2a, and 2b are in tables S1-S5: Solvent 30 min 4 h 24 h 48 h 72 h 1,2,4-trichlorobenzene S S S S S 1,2-dibromoethane PG PG S S S 2-butanone G CG CG CG CG 1,2-dichlorobenzene PG PG PG PG PG 1,3-dichlorobenzene S S S S S 1,4-dioxane G G G G CG 1-butanol S S G G G 1-pentanol G G G G G 1-propanol G G G G G 2-butanol G G G G G 2-Ethyl pyridine G G G G G 2-Picoline G G G G G 2-propanol G G G G G 3-chloro-1-propanol -

List of Criteria and Selected Air Contaminants to Be Used for Public Notice, with Spanish Translations. COMMON AIR CONTAMINANTS
List of Criteria and Selected Air Contaminants To Be Used for Public Notice, with Spanish Translations. COMMON AIR CONTAMINANTS: English Spanish NOX NOX (óxidos de nitrógeno) CO monóxido de carbono PM materia en partículas SO2 or SOX SO2 or SOX (óxidos de azufre) Halogens halógenos Hydrocarbons hidrocarburos Inorganic Acids ácidos inorgánicos Inorganic Bases bases inorgánicas Metals metales Carbon Compounds compuestos de carbono Carbon compounds including (but not limited to) : Benzene, Methyl Ethyl Ketone and Freon 11. Compuestos de carbono incluyendo (pero no limitándose a): Benceno, Metiletilcetona y Freón 11. OTHER AIR CONTAMINANTS English - Spanish Air Contaminant Glossary for Spanish Public Notice This list contains numerous air contaminants, mostly as listed in the ESL (= Effects Screening Levels) lists of 6/22/92 and updates of 1994. The Spanish translated names are capitalized for trademarks. In many such cases, there is no translation and the English word must be used. English Name Spanish Name Abamectin Abamectin Abate Abate acenaphthene acenafteno acetal acetal acetaldehyde acetaldehído acetamide acetamida acetic acid acido acético acetic anhydride anhídrido acético acetoacetoxyethyl methacrylate,2- 2-metacrilato acetoacetoxietílico acetone acetona acetone cyanohydrin cianohidrina de acetona acetonitrile acetonitrilo acetophenone acetofenona acetylaminofluorene,2- 2-acetilaminofluoreno acetyl chloride cloruro acetílico [AOAQG01/962 v2] Page 1 of 52 acetylene acetileno acetylene dichloride dicloruro de acetileno (1,2-dichloroethylene) -

The Fate of Arginine and Proline Carbon in Squid Tissuesl
Pacific Science (1982), vol. 36, no. 3 © 1983 by the University of Hawaii Press. All rights reserved The Fate of Arginine and Proline Carbon in Squid Tissuesl T. P. MOMMSEN,2 C. J. FRENCH,2 B. EMMETI,2 and P. W. HOCHACHKA2 ABSTRACT: The metabolism of proline and arginine was investigated in kidney, gill, and heart of the pelagic squid, Symplectoteuthis. The rates of CO2 release from 14C-proline exceeded the rates from 14C-arginine. The metabolic rate of arginine and proline was assessed by monitoring the incorporation of arginine-derived carbon into various intermediates. Arginine was metabolized, through ornithine, to proline as well as to glutamate and various subsequent derivatives (alanine, octopine, aspartate, and carboxylic acids). The same com ponents became labeled using 14C-proline as the starting substrate, but only the gill was capable ofconverting proline to arginine via the urea cycle. In addition, 14C-proline oxidation rates were high enough to exceed those of 14C-glucose in at least three tissues, kidney, heart, and inner mantle muscle. AT LEAST IN PART because ofthe large pool size data for heart, gill, and kidney from the squid, of free amino acids in cephalopod muscles Symplectoteuthis, showing the capacity for ar (e.g., see Hochachka, French, and Meredith ginine conversion to proline. The conversion 1978), interest recently has been focusing ofproline to arginine was measurable only in on their possible roles in energy metabolism. the gill. Although qualitatively similar to re During metabolic studies on the 1979 Alpha sults obtained with other species, these data Helix Cephalopod Expedition, relatively high also show some important, tissue-specific dif rates of CO2 release from arginine and ferences (Mommsen et al. -

Arginine Is Synthesized from Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates
0031-3998/11/6901-0046 Vol. 69, No. 1, 2011 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2010 International Pediatric Research Foundation, Inc. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates CHRIS TOMLINSON, MAHROUKH RAFII, MICHAEL SGRO, RONALD O. BALL, AND PAUL PENCHARZ Department of Paediatrics [C.T., M.S., P.P.], Research Institute [C.T., M.R., P.P.], The Hospital for Sick Children, Toronto, Ontario M5G1X8, Canada; Department of Nutritional Sciences [C.T., M.S., P.P.], University of Toronto, Toronto, Ontario M5S3E2, Canada; Department of Paediatrics [M.S.], St Michael’s Hospital, Toronto, Ontario M5B1W8, Canada; Department of Agricultural, Food and Nutritional Science [R.O.B., P.P.], University of Alberta, Edmonton, Alberta T6G2P5, Canada ABSTRACT: In neonatal mammals, arginine is synthesized in the litis (NEC) (8) and pulmonary hypertension (9). Furthermore, enterocyte, with either proline or glutamate as the dietary precursor. arginine supplementation was shown to reduce the incidence We have shown several times in piglets that proline is the only of all stages of NEC in moderately at risk infants (10) and a precursor to arginine, although in vitro evidence supports glutamate single bolus infusion of i.v. arginine improved oxygenation in in this role. Because of this uncertainty, we performed a multitracer infants with pulmonary hypertension (11). Therefore, because stable isotope study to determine whether proline, glutamate, or both are dietary precursors for arginine in enterally fed human neonates. arginine is clearly important for metabolism in the neonate, it Labeled arginine (M ϩ 2), proline (M ϩ 1), and glutamate (M ϩ 3) is critical to understand the metabolic pathways involved in its were given enterally to 15 stable, growing preterm infants (GA at synthesis. -

Locating and Estimating Sources of Ethylene Oxide
United States Office of Air Quality EPA-450/4-84-007L Environmental Protection Planning And Standards Agency Research Triangle Park, NC 27711 September 1986 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF ETHYLENE OXIDE L &E EPA- 450/4-84-007L September 1986 LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF ETHYLENE OXIDE U.S. Environmental Protection Agency Office of Air and Radiation Office of Air Quality Planning and Standards Research Triangle Park, North Carolina 27711 This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and approved for publication as received from the contractor. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, neither does mention of trade names or commercial products constitute endorsement or recommendation for use. EPA - 450/4-84-007L TABLE OF CONTENTS Section Page 1 Purpose of Document .......................................... 1 2 Overview of Document Contents ................................ 3 3 Background ................................................... 5 Nature of Pollutant .................................... 5 Overview of Production and Use ......................... 7 References for Section 3 .............................. 14 4 Emissions from Ethylene Oxide Production .................... 16 Ethylene Oxide Production ................................... 16 References for Section 4 .................................... 33 5 Emissions from Industries Which Use Ethylene