Essential Thrombocythaemia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activated Protein C Resistance: the Most Common Risk Factor for Venous Thromboembolism
J Am Board Fam Pract: first published as 10.3122/15572625-13-2-111 on 1 March 2000. Downloaded from CLINICAL REVIEW Activated Protein C Resistance: The Most Common Risk Factor for Venous Thromboembolism Dawn R. Sheppard, DO Background: Venous thromboembolism is a major cause of morbidity and mortality. Although activated protein C resistance (APC-R) is the most commonly recognized inherited risk factor for venous throm boembolism, little is known about its long-tenn implications on health. Methods: MEDLINE was searched from January 1989 through August 1999 using the key words ''thromboembolism," ''thrombosis," "activated protein C resistance," and "factor V Leiden." Results: One in 1000 people in the United States is affected by venous thromboembolism annually. APC-R is now understood to be responsible for up to 64% of these cases. APC-R, which occurs widely in some ethnic groups and is nearly absent in others, is due to a single point mutation in the gene for clot ting factor V. As a result, inactivation of factor V by activated protein C is impaired, leading to a hyper coagulable state. This condition creates a lifelong increased risk of thrombosis and, possibly, anticoag- ulant therapy.. Conclusion: Family physicians have a new tool for assessing risks for venous thromboembolism. Recognizing that up to 64% of patients with venous thromboembolism can have APe-R and treating this disorder with prophylactic and therapeutic anticoagulation might reduce patient morbidity and mortal ity from venous thromboembolism. Screening high-risk patients might now be indicated. (J Am Board Fam Pract 2000i13:111-5.) Venous thromboembolism, a serious health prob "thromboembolism," "thrombosis," "activated pro lem and an important cause of morbidity, affects tein C resistance," and "factor V Leiden." about 1 in 1000 persons annually. -

Familial Multiple Coagulation Factor Deficiencies
Journal of Clinical Medicine Article Familial Multiple Coagulation Factor Deficiencies (FMCFDs) in a Large Cohort of Patients—A Single-Center Experience in Genetic Diagnosis Barbara Preisler 1,†, Behnaz Pezeshkpoor 1,† , Atanas Banchev 2 , Ronald Fischer 3, Barbara Zieger 4, Ute Scholz 5, Heiko Rühl 1, Bettina Kemkes-Matthes 6, Ursula Schmitt 7, Antje Redlich 8 , Sule Unal 9 , Hans-Jürgen Laws 10, Martin Olivieri 11 , Johannes Oldenburg 1 and Anna Pavlova 1,* 1 Institute of Experimental Hematology and Transfusion Medicine, University Clinic Bonn, 53127 Bonn, Germany; [email protected] (B.P.); [email protected] (B.P.); [email protected] (H.R.); [email protected] (J.O.) 2 Department of Paediatric Haematology and Oncology, University Hospital “Tzaritza Giovanna—ISUL”, 1527 Sofia, Bulgaria; [email protected] 3 Hemophilia Care Center, SRH Kurpfalzkrankenhaus Heidelberg, 69123 Heidelberg, Germany; ronald.fi[email protected] 4 Department of Pediatrics and Adolescent Medicine, University Medical Center–University of Freiburg, 79106 Freiburg, Germany; [email protected] 5 Center of Hemostasis, MVZ Labor Leipzig, 04289 Leipzig, Germany; [email protected] 6 Hemostasis Center, Justus Liebig University Giessen, 35392 Giessen, Germany; [email protected] 7 Center of Hemostasis Berlin, 10789 Berlin-Schöneberg, Germany; [email protected] 8 Pediatric Oncology Department, Otto von Guericke University Children’s Hospital Magdeburg, 39120 Magdeburg, Germany; [email protected] 9 Division of Pediatric Hematology Ankara, Hacettepe University, 06100 Ankara, Turkey; Citation: Preisler, B.; Pezeshkpoor, [email protected] B.; Banchev, A.; Fischer, R.; Zieger, B.; 10 Department of Pediatric Oncology, Hematology and Clinical Immunology, University of Duesseldorf, Scholz, U.; Rühl, H.; Kemkes-Matthes, 40225 Duesseldorf, Germany; [email protected] B.; Schmitt, U.; Redlich, A.; et al. -
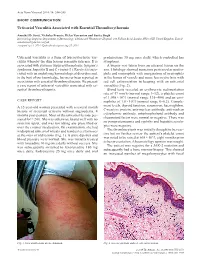
Urticarial Vasculitis Associated with Essential Thrombocythaemia
Acta Derm Venereol 2014; 94: 244–245 SHORT COMMUNICATION Urticarial Vasculitis Associated with Essential Thrombocythaemia Annabel D. Scott, Nicholas Francis, Helen Yarranton and Sarita Singh Dermatology Registrar, Department of Dermatology, Chelsea and Westminster Hospital, 369 Fulham Road, London SW10 9NH, United Kingdom. E-mail: [email protected] Accepted Apr 3, 2013; Epub ahead of print Aug 27, 2013 Urticarial vasculitis is a form of leucocytoclastic vas- prednisolone 30 mg once daily, which controlled her culitis whereby the skin lesions resemble urticaria. It is symptoms. associated with systemic lupus erythematosus, Sjögren’s A biopsy was taken from an uticarial lesion on the syndrome, hepatitis B and C viruses (1). Rarely it is asso- arm. Histology showed numerous perivascular neutro- ciated with an underlying haematological disorders and, phils and eosinophils with margination of neutrophils to the best of our knowledge, has never been reported in in the lumen of vessels and some leucocytoclasis with association with essential thrombocythaemia. We present red cell extravasation in keeping with an urticarial a case report of urticarial vasculitis associated with es- vasculitis (Fig. 2). sential thrombocythaemia. Blood tests revealed an erythrocyte sedimentation rate of 47 mm/h (normal range 1–12), a platelet count of 1,098 × 109/l (normal range 135–400) and an eosi- CASE REPORT nophilia of 1.0 × 109/l (normal range 0–0.2). Comple- A 32-year-old woman presented with a several month ment levels, thyroid function, serum iron, haemoglobin, history of recurrent urticaria without angioedema, 4 C-reactive protein, anti-nuclear antibody, anti-nuclear months post-partum. -

Regulation of Coagulation by the Fibrinolytic System: Expecting the Unexpected
Bleeding disorders Regulation of coagulation by the fibrinolytic system: expecting the unexpected K.A. Hajjar ABSTRACT Department of Cell and Recent investigations into fibrinolysis and coagulation have yielded exciting and unexpected findings. Developmental Biology, Of note, deficiencies of the major components of the classical fibrinolytic system, namely plasminogen, Department of Pediatrics tissue plasminogen activator, and urokinase, are now recognized to be rare causes of macrovascular Department of Medicine, thrombosis. At the same time, both plasminogen activator excess and fibrinolytic inhibitor deficiencies Weill Cornell Medical College, are associated with clinically significant bleeding, while elevated inhibitor levels, particularly thrombin New York, New York, USA activatable fibrinolysis inhibitor, appear to confer clinically significant risk for thrombosis. Receptor- mediated cell surface fibrinolysis adds a new dimension to the regulation of fibrin balance. Exaggerated expression of the annexin A2 complex, for example, is associated with hemorrhage in acute promyelo- Hematology Education: cytic leukemia, whereas autoantibodies directed against A2 correlate with thrombotic disease in the education program for the patients with antiphospholipid syndrome. The contributions of the urokinase receptor and a diverse annual congress of the European array of plasminogen receptors to fibrin homeostasis remain to be defined. Future studies are likely to Hematology Association reveal novel mechanisms that co-regulate coagulation and -

Alpha-Dystroglycan Plays Functional Roles in Platelet Aggregation and Thrombus Growth
Alpha-dystroglycan plays functional roles in platelet aggregation and thrombus growth by Reid Gallant A thesis submitted in conformity with the requirements For the degree of Master of Science Graduate Department of Laboratory Medicine and Pathobiology University of Toronto © Copyright by Reid Gallant 2017 i Alpha-dystroglycan Plays Functional Roles in Platelet Aggregation and Thrombus Growth Reid Gallant Master of Science Department of Laboratory Medicine and Pathobiology University of Toronto 2017 ABSTRACT Fibrinogen (Fg) and von Willebrand factor (VWF) have been considered essential for platelet adhesion and aggregation. However, platelet aggregation still occurs in mice lacking Fg and/or VWF but not β3 integrin, suggesting other, unidentified αIIbβ3 integrin ligand(s) mediate platelet aggregation. Through screening published platelet proteomics data, we identified a candidate, alpha-dystroglycan (α-DG). Using Western blot and flow cytometry, I found α-DG is expressed on platelets. Using aggregometry, I observed that antibodies against α-DG or its N- terminal Laminin-binding site, decreased platelet aggregation induced by various platelet agonists in both platelet-rich plasma and gel-filtered platelets. These antibodies also decreased platelet adhesion/aggregation in perfusion chambers independent of α-DG-Laminin interaction. Using laser injury intravital microscopy and carotid artery thrombosis models, we further found that these anti-α-DG antibodies decreased thrombus growth in vivo. Our results showed that α- DG may form an α-DG-fibronectin complex that binds to αIIbβ3 integrin, contributing to platelet adhesion/aggregation, and thrombosis growth. ii Acknowledgements ―It helps a man immensely to be a bit of a hero-worshipper, and the stories of the lives of the masters of medicine do much to stimulate our ambition and rouse our sympathies‖ – Sir William Osler I will always be grateful to my MSc supervisor, Dr. -

Factor V Leiden Thrombophilia
Factor V Leiden thrombophilia Description Factor V Leiden thrombophilia is an inherited disorder of blood clotting. Factor V Leiden is the name of a specific gene mutation that results in thrombophilia, which is an increased tendency to form abnormal blood clots that can block blood vessels. People with factor V Leiden thrombophilia have a higher than average risk of developing a type of blood clot called a deep venous thrombosis (DVT). DVTs occur most often in the legs, although they can also occur in other parts of the body, including the brain, eyes, liver, and kidneys. Factor V Leiden thrombophilia also increases the risk that clots will break away from their original site and travel through the bloodstream. These clots can lodge in the lungs, where they are known as pulmonary emboli. Although factor V Leiden thrombophilia increases the risk of blood clots, only about 10 percent of individuals with the factor V Leiden mutation ever develop abnormal clots. The factor V Leiden mutation is associated with a slightly increased risk of pregnancy loss (miscarriage). Women with this mutation are two to three times more likely to have multiple (recurrent) miscarriages or a pregnancy loss during the second or third trimester. Some research suggests that the factor V Leiden mutation may also increase the risk of other complications during pregnancy, including pregnancy-induced high blood pressure (preeclampsia), slow fetal growth, and early separation of the placenta from the uterine wall (placental abruption). However, the association between the factor V Leiden mutation and these complications has not been confirmed. Most women with factor V Leiden thrombophilia have normal pregnancies. -

Factor V Leiden Thrombophilia Jody Lynn Kujovich, MD
GENETEST REVIEW Genetics in Medicine Factor V Leiden thrombophilia Jody Lynn Kujovich, MD TABLE OF CONTENTS Pathogenic mechanisms and molecular basis.................................................2 Obesity ...........................................................................................................8 Prevalence..............................................................................................................2 Surgery...........................................................................................................8 Diagnosis................................................................................................................2 Thrombosis not convincingly associated with Factor V Leiden....................8 Clinical diagnosis..............................................................................................2 Arterial thrombosis...........................................................................................8 Testing................................................................................................................2 Myocardial infarction.......................................................................................8 Indications for testing......................................................................................3 Stroke .................................................................................................................8 Natural history and clinical manifestations......................................................3 Genotype-phenotype -

Appendix Search Strategy Treatment of Hemophilia.Pdf
Appendix Search strategies Hemophilia – general aspects PubMed (NLM) September 2009 Von Willebrand disease (TiAb) AND Controlled clinical trial (PT) NOT Purpura, Thrombocytopenic (Me) Angiohemophilia (TiAb) Meta analysis (PT) Blood coagulation disorders (Me) Randomized controlled trial (PT) Hemophilia (TiAb) Systematic (SB) Haemophilia (TiAb) Bleeding disorder (TiAb) Random* (Ti) Bleeding disorders (TiAb) OR Control* (Ti) NOT Medline (SB) ("controlled clinical trial"[Publication Type] OR "meta analysis"[Publication Type] OR "randomized controlled trial"[Publication Type] OR systematic[sb] OR ((random*[Title] OR control*[Title]) NOT Medline[sb])) AND ("von Willebrand Disease"[title/abstract] OR "angiohemophilia"[title/Abstract] OR "Blood Coagulation Disorders"[Mesh terms] OR "hemophilia"[title/abstract] OR "haemophilia"[title/abstract] OR "bleeding disorder"[title/abstract] OR "bleeding disorders"[Title/abstract]) NOT "Purpura, Thrombocytopenic"[MeSH Terms] 211 Hemophilia – general aspects Embase.com (Elsevier) September 2009 Blood clotting factor deficiency (Exp,MJR) AND Clinical trial (Exp) NOT Thrombocytopenic purpura (Exp) Von Willebrand disease (Ti) Intervention study (De) Angiohemophilia (Ti) Longitudinal study (De) Angiohaemophilia (Ti) Prospective study (De) Hemophilia (Ti) Meta analysis (De) Haemophilia (Ti) Systematic review (De) Bleeding disorder (Ti) Random* (Ti) Bleeding disorders (Ti) Control* (Ti) ('blood clotting factor deficiency'/exp/mjOR 'von willebrand disease':ti OR 'angiohemophilia':ti OR 'angiohaemophilia':ti OR 'hemophilia':ti -

Immune Thrombocytopenic Purpura with Subsequent Development of JAK2 V617F-Positive Essential Thrombocythemia: Case Report
ISSN: 2640-7914 DOI: https://dx.doi.org/10.17352/ahcrr CLINICAL GROUP Received: 13 July, 2021 Case Report Accepted: 03 August, 2021 Published: 04 August, 2021 *Corresponding author: Marisabel Hurtado-Castillo, Immune thrombocytopenic PGY-3, Internal Medicine, Department of Medicine at NYU, 536 Ovington Avenue Apartment 3, Brooklyn NYC 11209, USA, Tel: 718-312-9641; purpura with subsequent Email: Keywords: Immune thrombocytopenic purpura; development of JAK2 Essential thrombocythemia; JAK-STAT signaling path- way; JAK2(V617F) mutation V617F-positive essential https://www.peertechzpublications.com thrombocythemia: Case Report Marisabel Hurtado-Castillo1*, Brian Flaherty2 and Morris Jrada2 1PGY-3, Internal Medicine, Department of Medicine at NYU, Brooklyn, NY, USA 2Clinical Assistant Professor, Department of Medicine at NYU Grossman School of Medicine, Brooklyn, NY, USA Abstract The sequential occurrence of Immune Thrombocytopenic Purpura (ITP) and Essential Thrombocythemia (ET) has been reported in the literature on a few occasions, as these are two hematologic disorders with distinct etiologies and patients usually have contrasting clinical presentations. Our case highlights the sequential occurrence of ITP, followed by Janus kinase 2 (JAK2) (V617F)-positive ET in a 64-year-old white woman, after four years of follow-up. The pathophysiology relating to these two conditions is incompletely understood, however, JAK2(V617F) mutation has been found in all the cases reported. Early identifi cation of JAK2(V617F) mutation in a patient with a -

Thrombocytopenia.Pdf
THROMBOCYTOPENIA DIFFERENTIAL DIAGNOSIS FALSELY LOW PLATELET COUNT In vitro platelet clumping caused by EDTA-dependent agglutinins Giant platelets COMMON CAUSES OF THROMBOCYTOPENIA Pregnancy (gestational thrombocytopenia, preeclampsia) Drug-induced thrombocytopenia (i.e., heparin, quinidine, quinine, and sulfonamides) Viral infections (ie. HIV, rubella, infectious mononucleosis) Hypersplenism due to chronic liver disease Dilutional (massive transfusion) OTHER CAUSES OF THROMBOCYTOPENIA Myelodysplasia Congenital thrombocytopenia Thrombotic thrombocytopenic purpura (TTP) -hemolytic-uremic syndrome (HUS) Chronic disseminated intravascular coagulation (DIC) Autoimmune diseases, such as systemic lupus erythematosus-associated lymphoproliferative disorders (CLL and NHL) Sepsis Idiopathic thrombocytopenic purpura (ITP)* DIFFERENTIAL FOR THROMBOCYTOPENIA BASED ON CLINICAL SETTING CLINICAL SETTING DIFFERENTIAL DIAGNOSES Cardiac surgery Cardiopulmonary bypass, HIT, dilutional thrombocytopenia, PTP Interventional cardiac Abciximab or other IIb/IIIa blockers, HIT procedure Sepsis syndrome DIC, ehrlichiosis, sepsis, hemophagocytosis syndrome, drug-induced, misdiagnosed TTP, mechanical ventilation, pulmonary artery catheters Pulmonary failure DIC, hantavirus pulmonary syndrome, mechanical ventilation, pulmonary artery catheters Mental status TTP, ehrlichiosis changes/seizures Renal failure TTP, Dengue, HIT, DIC, HUS Continuous hemofiltration HIT, consumption by filter and tubing Cardiac failure HIT, drug-induced, pulmonary artery catheter Post-surgery -

Congenital Thrombophilia
Congenital thrombophilia The term congenital thrombophilia covers a range of Factor V Leiden and pregnancy conditions that are inherited by someone at birth. This means It is important that women with Factor V Leiden who are that their blood is sticker than normal, which increases the pregnant discuss this with their obstetrician as they have an risk of blood clots and thrombosis. increased risk of venous thrombosis during pregnancy. Some Factor V Leiden and Prothrombin 20210 are the most evidence suggests that they may also have a slightly higher common thrombophilias among people of European origin. risk of miscarriage and placental problems. Other congenital thrombophilias include Protein C Deficiency, Protein S Deficiency, and Antithrombin Deficiency. Prothrombin 20210 Prothrombin is one of the blood clotting factors. It circulates Factor V Leiden in the blood and when activated, is converted to thrombin. Factor V Leiden is by far the most common congenital Thrombin causes fibrinogen, another clotting factor, to thrombophilia. In the UK it is present in 1 in 20 individuals of convert to fibrin strands, which make up part of a clot. European origin. It is rare in people of Black or Asian origin. The condition known as Prothrombin 20210 is due to a Factor V Leiden is caused by a change in the gene for Factor mutation of the prothrombin gene. Individuals with the V, which helps the blood to clot. To stop a clot spreading a condition tend to have slightly stickier blood, due to higher natural blood thinner, known as Protein C, breaks down prothrombin levels. Factor V. -

Original Article Endogenous Risk Factors for Deep-Vein Thrombosis in Patients with Acute Spinal Cord Injuries
Spinal Cord (2007) 45, 627–631 & 2007 International Spinal Cord Society All rights reserved 1362-4393/07 $30.00 www.nature.com/sc Original Article Endogenous risk factors for deep-vein thrombosis in patients with acute spinal cord injuries S Aito*,1, R Abbate2, R Marcucci2 and E Cominelli1 1Spinal Unit, Careggi University Hospital, Florence, Italy; 2Medical division, coagulation disease, Careggi University Hospital, Florence, Italy Study design: Case–control study. Aim of the study: Investigate the presence of additional endogenous risk factors of deep-vein thrombosis (DVT). Setting: Regional Spinal Unit of Florence, Italy. Methods: A total of 43 patients with spinal lesion and a history of DVT during the acute stage of their neurological impairment (Group A) were comprehensively evaluated and the blood concentrations of the following risk factors, that are presumably associated with DVT, were determined: antithrombin III (ATIII), protein C (PC), protein S (PS), factor V Leiden, gene 200210A polymorphism, homocysteine (Hcy), inhibitor of plasminogen activator-1 (PAI-1) and lipoprotein A (LpA). The control group (Group B) consisted of 46 patients matched to Group A for sex, age, neurological status and prophylactic treatment during the acute stage, with no history of DVT. Statistical analysis was performed using the Mann–Whitney and Fisher’s exact tests. Results: Of the individuals in GroupA, 14% had no risk factor and 86% had at least one; however, in GroupB 54% had no endogenous risk factors and 46% had at least one. None of the individuals in either grouphad a deficit in their coagulation inhibitors (ATIII, PC and PS), and the LpA level was equivalent in the two groups.