Thrombocytopenia.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protein S Deficiency Presenting with Hemorrhage in a Term Neonate
: Curre re nt a R C e Ayari et al., Health Care Current Reviews 2018, 6:1 h v t i l e a w DOI: 10.4172/2375-4273.1000219 e s H Health Care: Current Reviews ISSN: 2375-4273 Review Article Open Access Protein S Deficiency Presenting with Hemorrhage in a Term Neonate Fairouz Ayari*, Takoua Bensmail, Essid Latifa, Wiem Barbaria and Samia Kacem Neonatology Intensive Care Unit of the Maternity and Neonatology Center, Tunis, Tunisia Abstract Unexplained bleeding symptoms in otherwise healthy full-term usually present a diagnostic challenge for treating physicians requiring prompt and accurate laboratory investigations to ensure appropriate treatment and possibly avoid long-term morbidity. We report a case of a term neonate with severe protein S deficiency manifested by systemic hemorrhage and multiple organ failure at 9 days of age. We review how protein S influences the coagulation and the fibrinolytic pathways, discussing therapeutic approaches of neonates with purpura fulminans. Keywords: Protein S deficiency; Blood sample; Thrombophilic dis- resuscitation with 20 ml/kg bodyweight (BW) saline solution and, after order blood sampling, intravenous administration of 10 mg vitamin K, 20 ml/kg BW fresh frozen plasma, 20 ml/kg BW packed red blood cells Introduction (5 transfusion cycles), 20 mg/kg BW Phenobarbital and vasoactive Protein S (PS) is an antithrombotic plasma protein that acts mainly drugs. Cerebral ultrasound revealed intraventricular haemorrhage, as a cofactor of activated protein C (APC) anticoagulant activity in the abdominal ultrasound showed splenic hemorrhage and cardiac degradation of factor Va and activated factor VIII [1]. PS circulates in ultrasound showed a floating intracardiac thrombus. -

Thrombotic Thrombocytopenic Purpura and Systemic Lupus Erythematosus: Successful Management of a Rare Presentation
Indian J Crit Care Med July-September 2008 Vol 12 Issue 3 Case Report Thrombotic thrombocytopenic purpura and systemic lupus erythematosus: Successful management of a rare presentation Pratish George, Jasmine Das, Basant Pawar1, Naveen Kakkar2 Thrombotic thrombocytopenic purpura (TTP) and systemic lupus erythematosus (SLE) very rarely present simultaneously and pose a diagnostic and therapeutic dilemma to the critical care team. Prompt diagnosis and management with plasma exchange and immunosuppression is life-saving. A patient critically ill with TTP and SLE, successfully managed in the acute period of illness with plasma exchange, steroids and Abstract mycophenolate mofetil is described. Key words: Plasma exchange, systemic lupus erythematosus, thrombotic thrombocytopenic purpura Introduction Case Report Systemic lupus erythematosis (SLE) is diagnosed by A 30-year-old lady was admitted with fever and the presence of four or more of the following criteria, jaundice. A week earlier she had undergone an serially or simultaneously: malar rash, discoid rash, uncomplicated medical termination of pregnancy at photosensitivity, oral ulcers, non erosive arthritis, serositis, another hospital, at 13 weeks of gestation. She had an renal abnormalities including proteinuria or active urinary uneventful pregnancy with twins two years earlier and sediments, neuropsychiatric features, hematological the twins were diagnosed to have thalassemia major. abnormalities including hemolytic anemia, leucopenia, She was subsequently diagnosed to have thalassemia lymphopenia and thrombocytopenia, immunological minor and her husband had thalassemia minor trait. No markers like anti-ds DNA or anti-Smith antibody and high earlier history of spontaneous Þ rst trimester abortions Antinuclear antibody titres. Thrombotic thrombocytopenic was present. purpura (TTP) in patients with SLE is extremely rare. -
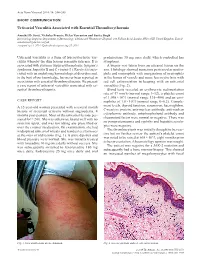
Urticarial Vasculitis Associated with Essential Thrombocythaemia
Acta Derm Venereol 2014; 94: 244–245 SHORT COMMUNICATION Urticarial Vasculitis Associated with Essential Thrombocythaemia Annabel D. Scott, Nicholas Francis, Helen Yarranton and Sarita Singh Dermatology Registrar, Department of Dermatology, Chelsea and Westminster Hospital, 369 Fulham Road, London SW10 9NH, United Kingdom. E-mail: [email protected] Accepted Apr 3, 2013; Epub ahead of print Aug 27, 2013 Urticarial vasculitis is a form of leucocytoclastic vas- prednisolone 30 mg once daily, which controlled her culitis whereby the skin lesions resemble urticaria. It is symptoms. associated with systemic lupus erythematosus, Sjögren’s A biopsy was taken from an uticarial lesion on the syndrome, hepatitis B and C viruses (1). Rarely it is asso- arm. Histology showed numerous perivascular neutro- ciated with an underlying haematological disorders and, phils and eosinophils with margination of neutrophils to the best of our knowledge, has never been reported in in the lumen of vessels and some leucocytoclasis with association with essential thrombocythaemia. We present red cell extravasation in keeping with an urticarial a case report of urticarial vasculitis associated with es- vasculitis (Fig. 2). sential thrombocythaemia. Blood tests revealed an erythrocyte sedimentation rate of 47 mm/h (normal range 1–12), a platelet count of 1,098 × 109/l (normal range 135–400) and an eosi- CASE REPORT nophilia of 1.0 × 109/l (normal range 0–0.2). Comple- A 32-year-old woman presented with a several month ment levels, thyroid function, serum iron, haemoglobin, history of recurrent urticaria without angioedema, 4 C-reactive protein, anti-nuclear antibody, anti-nuclear months post-partum. -

081999 Disseminated Intravascular Coagulation
The New England Journal of Medicine Current Concepts Systemic activation+ of coagulation DISSEMINATED INTRAVASCULAR COAGULATION Intravascular+ Depletion of platelets+ deposition of fibrin and coagulation factors MARCEL LEVI, M.D., AND HUGO TEN CATE, M.D. Thrombosis of small+ Bleeding and midsize vessels+ ISSEMINATED intravascular coagulation is and organ failure characterized by the widespread activation Dof coagulation, which results in the intravas- Figure 1. The Mechanism of Disseminated Intravascular Coag- cular formation of fibrin and ultimately thrombotic ulation. occlusion of small and midsize vessels.1-3 Intravascu- Systemic activation of coagulation leads to widespread intra- lar coagulation can also compromise the blood sup- vascular deposition of fibrin and depletion of platelets and co- agulation factors. As a result, thrombosis of small and midsize ply to organs and, in conjunction with hemodynam- vessels may occur, contributing to organ failure, and there may ic and metabolic derangements, may contribute to be severe bleeding. the failure of multiple organs. At the same time, the use and subsequent depletion of platelets and coag- ulation proteins resulting from the ongoing coagu- lation may induce severe bleeding (Fig. 1). Bleeding may be the presenting symptom in a patient with disseminated intravascular coagulation, a factor that can complicate decisions about treatment. TABLE 1. COMMON CLINICAL CONDITIONS ASSOCIATED WITH DISSEMINATED ASSOCIATED CLINICAL CONDITIONS INTRAVASCULAR COAGULATION. AND INCIDENCE Sepsis Infectious Disease Trauma Serious tissue injury Disseminated intravascular coagulation is an ac- Head injury Fat embolism quired disorder that occurs in a wide variety of clin- Cancer ical conditions, the most important of which are listed Myeloproliferative diseases in Table 1. -

What Everyone Should Know to Stop Bleeding After an Injury
What Everyone Should Know to Stop Bleeding After an Injury THE HARTFORD CONSENSUS The Joint Committee to Increase Survival from Active Shooter and Intentional Mass Casualty Events was convened by the American College of Surgeons in response to the growing number and severity of these events. The committee met in Hartford Connecticut and has produced a number of documents with rec- ommendations. The documents represent the consensus opinion of a multi-dis- ciplinary committee involving medical groups, the military, the National Security Council, Homeland Security, the FBI, law enforcement, fire rescue, and EMS. These recommendations have become known as the Hartford Consensus. The overarching principle of the Hartford Consensus is that no one should die from uncontrolled bleeding. The Hartford Consensus recommends that all citizens learn to stop bleeding. Further information about the Hartford Consensus and bleeding control can be found on the website: Bleedingcontrol.org 2 SAVE A LIFE: What Everyone Should Know to Stop Bleeding After an Injury Authors: Peter T. Pons, MD, FACEP Lenworth Jacobs, MD, MPH, FACS Acknowledgements: The authors acknowledge the contributions of Michael Cohen and James “Brooks” Hart, CMI to the design of this manual. Some images adapted from Adam Wehrle, EMT-P and NAEMT. © 2017 American College of Surgeons CONTENTS SECTION 1 3 ■ Introduction ■ Primary Principles of Trauma Care Response ■ The ABCs of Bleeding SECTION 2 5 ■ Ensure Your Own Safety SECTION 3 6 ■ A – Alert – call 9-1-1 SECTION 4 7 ■ B – Bleeding – find the bleeding injury SECTION 5 9 ■ C – Compress – apply pressure to stop the bleeding by: ■ Covering the wound with a clean cloth and applying pressure by pushing directly on it with both hands, OR ■Using a tourniquet, OR ■ Packing (stuff) the wound with gauze or a clean cloth and then applying pressure with both hands SECTION 6 13 ■ Summary 2 SECTION 1: INTRODUCTION Welcome to the Stop the Bleed: Bleeding Control for the Injured information booklet. -

Immune-Pathophysiology and -Therapy of Childhood Purpura
Egypt J Pediatr Allergy Immunol 2009;7(1):3-13. Review article Immune-pathophysiology and -therapy of childhood purpura Safinaz A Elhabashy Professor of Pediatrics, Ain Shams University, Cairo Childhood purpura - Overview vasculitic disorders present with palpable Purpura (from the Latin, purpura, meaning purpura2. Purpura may be secondary to "purple") is the appearance of red or purple thrombocytopenia, platelet dysfunction, discolorations on the skin that do not blanch on coagulation factor deficiency or vascular defect as applying pressure. They are caused by bleeding shown in table 1. underneath the skin. Purpura measure 0.3-1cm, A thorough history (Table 2) and a careful while petechiae measure less than 3mm and physical examination (Table 3) are critical first ecchymoses greater than 1cm1. The integrity of steps in the evaluation of children with purpura3. the vascular system depends on three interacting When the history and physical examination elements: platelets, plasma coagulation factors suggest the presence of a bleeding disorder, and blood vessels. All three elements are required laboratory screening studies may include a for proper hemostasis, but the pattern of bleeding complete blood count, peripheral blood smear, depends to some extent on the specific defect. In prothrombin time (PT) and activated partial general, platelet disorders manifest petechiae, thromboplastin time (aPTT). With few exceptions, mucosal bleeding (wet purpura) or, rarely, central these studies should identify most hemostatic nervous system bleeding; -

Outcomes of Patients with Thrombocytopenia Evaluated at Hematology Subspecialty Clinics
Henry Ford Health System Henry Ford Health System Scholarly Commons Hematology Oncology Articles Hematology-Oncology 2-11-2021 Outcomes of patients with thrombocytopenia evaluated at hematology subspecialty clinics Zaid H. Abdel Rahman Kevin C. Miller H Jabbour Yaser Alkhatib Vijayalakshmi Donthireddy Follow this and additional works at: https://scholarlycommons.henryford.com/ hematologyoncology_articles Hematol Oncol Stem Cell Ther xxx (xxxx) xxx Available at www.sciencedirect.com ScienceDirect journal homepage: www.elsevier.com/locate/hemonc Outcomes of patients with thrombocytopenia evaluated at hematology subspecialty clinics Zaid H. Abdel Rahman a,*, Kevin C. Miller b, Hiba Jabbour c, Yaser Alkhatib c, Vijaya Donthireddy c a Division of Hematology and Medical Oncology, Mayo Clinic, Jacksonville, FL, USA b Department of Medicine, Massachusetts General Hospital, Boston, MA, USA c Division of Hematology and Medical Oncology, Henry Ford Hospital, Detroit, MI, USA Received 6 October 2020; received in revised form 9 December 2020; accepted 15 January 2021 KEYWORDS Abstract Hematology; Background: Thrombocytopenia is a frequently encountered laboratory abnormality and a Malignancy; common reason for hematology referrals. Workup for thrombocytopenia is not standardized Platelets; and frequently does not follow an evidence-based algorithm. We conducted a systematic anal- Referrals; Thrombocytopenia ysis to evaluate the laboratory testing and outcomes of patients evaluated for thrombocytope- nia at hematology clinics in a tertiary referral center between 2013 and 2016. Patient and methods: We performed a comprehensive chart review for patients evaluated for thrombocytopenia during the study period. Patients were followed for 1 year from the initial hematology evaluation and assessed for the development of a hematologic malignancy, rheumatologic, or infectious diseases among other clinical outcomes. -

Severe Fever with Thrombocytopenia Syndrome: a Newly Discovered Emerging Infectious Disease
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector REVIEW Severe fever with thrombocytopenia syndrome: a newly discovered emerging infectious disease D. X. Li Key Laboratory for Medical Virology, National Institute for Viral Disease Control and Prevention, China CDC, Beijing, China Abstract Severe fever with thrombocytopenia syndrome (SFTS) is a newly discovered emerging infectious disease that has recently become epidemic in Asia. The causative agent of SFTS is a novel phlebovirus in the family Bunyaviridae, designated SFTS virus (SFTSV). SFTS clinically presents with high fever, thrombocytopenia, leukocytopenia, gastrointestinal disorders, and multi-organ dysfunction, with a high viral load and a high case- fatality rate. In human infection, SFTSV targets microphages, replicates in the spleen of infected mice, and causes thrombocytopenia and a cytokine storm. The tick disseminates virus to humans and animals, forming a special transmission model in nature. Person-to-person transmission though direct contact with patient blood has been frequently reported. Measurements of viral RNA and antibodies have been established for diagnosis, but vaccines and specific therapeutics are not available so far. Clinical Microbiology and Infection © 2015 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved. Keywords: Clinical, epidemiology, SFTS virus, SFTS, virology Article published online: 11 March 2015 Virology D.X. Li, Key Laboratory for Medical Virology, NCHFP, RRC, National Institute for Viral Disease Control and Prevention, China CDC, Bei- jing 102206, China E-mail: [email protected] The causative agent of SFTS is SFTSV, which is a tick-borne virus in the family Bunyaviridae, genus Phlebovirus. -
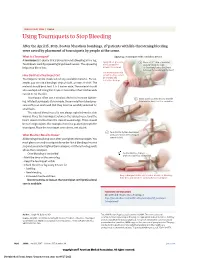
Using Tourniquets to Stop Bleeding
JAMA PATIENT PAGE | Trauma Using Tourniquets to Stop Bleeding After the April 15, 2013, Boston Marathon bombings, 27 patients with life-threatening bleeding were saved by placement of tourniquets by people at the scene. What Is a Tourniquet? Applying a tourniquet with a windlass device A tourniquet is a device that is placed around a bleeding arm or leg. Apply direct pressure 1 Place a 2-3” strip of material Tourniquets work by squeezing large blood vessels. The squeezing to the wound for about 2” from the edge helps stop blood loss. at least 15 minutes. of the wound over a long bone between the wound and the heart. Use a tourniquet only How Do I Put a Tourniquet On? when bleeding cannot be stopped and Tourniquets can be made out of any available material. For ex- is life threatening. ample, you can use a bandage, strip of cloth, or even a t-shirt. The material should be at least 2 to 3 inches wide. The material should also overlap itself. Using thin straps or material less than 2 inches wide can rip or cut the skin. Tourniquets often use a windlass device to increase tighten- 2 Insert a stick or other strong, straight ing. Inflated tourniquets (for example, those made from blood pres- item into the knot to act as a windlass. sure cuffs) can work well. But they must be carefully watched for small leaks. The injured blood vessel is not always right below the skin wound. Place the tourniquet between the injured vessel and the heart, about 2 inches from the closest wound edge. -

Neonatal Leukopenia and Thrombocytopenia
Neonatal Leukopenia and Thrombocytopenia Vandy Black, M.D., M.Sc., FAAP March 3, 2016 April 14, 2011 Objecves • Summarize the differenHal diagnosis of leukopenia and/or thrombocytopenia in a neonate • Describe the iniHal steps in the evaluaon of a neonate with leukopenia and/or thrombocytopenia • Review treatment opHons for leukopenia and/ or thrombocytopenia in the NICU Clinical Case 1 • One day old male infant admiUed to the NICU for hypoglycemia and a sepsis rule out • Born at 38 weeks EGA by SVD • Birth weight 4 lbs 13 oz • Exam shows a small cephalohematoma; no dysmorphic features • PLT count 42K with an otherwise normal CBC Definions • Normal WBC count 9-30K at birth – Mean 18K • What is the ANC and ALC – <1000/mm3 is abnormal – 6-8% of infants in the NICU • Normal platelet count: 150-450,000/mm3 – Not age dependent – 22-35% of infants in the NICU have plts<150K Neutropenia Absolute neutrophil count <1500/mm3 Category ANC* InfecHon risk • Mild 1000-1500 None • Moderate 500-1000 Minimal • Severe <500 Moderate to Severe (Highest if <200) • Recurrent bacterial or fungal infecHons are the hallmark of symptomac neutropenia! • *ANC = WBC X % (PMNs + Bands) / 100 DefiniHon of Neutropenia Black and Maheshwari, Neoreviews 2009 How to Approach Cytopenias • Normal vs. abnormal (consider severity) • Malignant vs. non-malignant • Congenital vs. acquired • Is the paent symptomac • Transient, recurrent, cyclic, or persistent How to Approach Cytopenias • Adequate vs. decreased marrow reserve • Decreased producHon vs. increased destrucHon/sequestraon Decreased neutrophil/platelet producon • Primary – Malignancy/leukemia/marrow infiltraon – AplasHc anemia – Genec disorders • Secondary – InfecHous – Drug-induced – NutriHonal • B12, folate, copper Increased destrucHon/sequestraon • Immune-mediated • Drug-induced • Consumpon à Hypersplenism vs. -

ER Guide to Bleeding Disorders
Bleeding disorders ER guide to bleeding disorders 1 Table of contents 4 General Guidelines 4–5 national Hemophilia Foundation guidelines 5–10 Treatment options 10 HemopHilia a Name:__________________________________________________________________________________________________ 10–11 national Hemophilia Foundation guidelines Address:________________________________________________________________________________________________ 12 dosage chart Phone:__________________________________________________________________________________________________ 14–15 Treatment products 16 HemopHilia B In case of emergency, contact: ______________________________________________________________________________ 16 national Hemophilia Foundation guidelines Relation to patient:________________________________________________________________________________________ 17 dosage chart 18 Treatment products 19 HemopHilia a or B with inHiBiTors Diagnosis: Hemophilia A: Mild Moderate Severe 20 national Hemophilia Foundation guidelines Inhibitors Inhibitors Bethesda units (if known) ____________________________________ 21 Treatment products Hemophilia B: Mild Moderate Severe 22–23 Von willeBrand disease Inhibitors Inhibitors Bethesda units (if known) ____________________________________ 23–24 national Hemophilia Foundation guidelines von Willebrand disease: Type 1 Type 2 Type 3 Platelet type 25 Treatment products 27 Bibliography Preferred product:_________________________________________________________________________________________ Dose for life-threatening -

Appendix Search Strategy Treatment of Hemophilia.Pdf
Appendix Search strategies Hemophilia – general aspects PubMed (NLM) September 2009 Von Willebrand disease (TiAb) AND Controlled clinical trial (PT) NOT Purpura, Thrombocytopenic (Me) Angiohemophilia (TiAb) Meta analysis (PT) Blood coagulation disorders (Me) Randomized controlled trial (PT) Hemophilia (TiAb) Systematic (SB) Haemophilia (TiAb) Bleeding disorder (TiAb) Random* (Ti) Bleeding disorders (TiAb) OR Control* (Ti) NOT Medline (SB) ("controlled clinical trial"[Publication Type] OR "meta analysis"[Publication Type] OR "randomized controlled trial"[Publication Type] OR systematic[sb] OR ((random*[Title] OR control*[Title]) NOT Medline[sb])) AND ("von Willebrand Disease"[title/abstract] OR "angiohemophilia"[title/Abstract] OR "Blood Coagulation Disorders"[Mesh terms] OR "hemophilia"[title/abstract] OR "haemophilia"[title/abstract] OR "bleeding disorder"[title/abstract] OR "bleeding disorders"[Title/abstract]) NOT "Purpura, Thrombocytopenic"[MeSH Terms] 211 Hemophilia – general aspects Embase.com (Elsevier) September 2009 Blood clotting factor deficiency (Exp,MJR) AND Clinical trial (Exp) NOT Thrombocytopenic purpura (Exp) Von Willebrand disease (Ti) Intervention study (De) Angiohemophilia (Ti) Longitudinal study (De) Angiohaemophilia (Ti) Prospective study (De) Hemophilia (Ti) Meta analysis (De) Haemophilia (Ti) Systematic review (De) Bleeding disorder (Ti) Random* (Ti) Bleeding disorders (Ti) Control* (Ti) ('blood clotting factor deficiency'/exp/mjOR 'von willebrand disease':ti OR 'angiohemophilia':ti OR 'angiohaemophilia':ti OR 'hemophilia':ti