ER Guide to Bleeding Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Photoaging & Skin Damage
Use_for_Revised_OFC_Only_2006_PhotoagingSkinDamage 5/21/13 9:11 AM Page 2 PEORIA (309) 674-7546 MORTON (309) 263-7546 GALESBURG (309) 344-5777 PERU (815) 224-7400 NORMAL (309) 268-9980 CLINTON, IA (563) 242-3571 DAVENPORT, IA (563) 344-7546 SoderstromSkinInstitute.comsoderstromskininstitute.com FROMFrom YOUR Your DERMATOLOGISTDermatologist [email protected]@skinnews.com PHOTOAGING & SKIN DAMAGE Before You Worship The Sun Who’s At Risk? Today, many researchers and dermatologists Skin types that burn easily and tan rarely are believe that wrinkling and aging changes of the skin much more susceptible to the ravages of the sun on the are much more related to sun damage than to age! skin than are those that tan easily, rather than burn. Many of the signs of skin damage from the sun are Light complected, blue-eyed, red-haired people such as pictured on these pages. The decrease in the ozone Swedish, Irish, and English, are usually more suscep- layer, increasing the sun’s intensity, and the increasing tible to photo damage, and their skin shows the signs sun exposure among our population – through work, of photo damage earlier in life and in a more pro- sports, sunbathing and tanning parlors – have taken a nounced manner. Dark complexions give more protec- tremendous toll on our skin. Sun damage to the skin tion from light and the sun. ranks with other serious health dangers of smoking, alcohol, and increased cholesterol, and is being seen in younger and younger people. NO TAN IS A SAFE TAN! Table of Contents Sun Damage .............................................Pg. 1 Skin Cancer..........................................Pgs. 2-3 Mohs Micrographic Surgery ......................Pg. -

Clinical Commissioning Policy: Susoctocog Alfa for Treating Bleeding Episodes in People with Acquired Haemophilia a (All Ages)
Clinical Commissioning Policy: Susoctocog alfa for treating bleeding episodes in people with acquired haemophilia A (all ages) NHS England Reference: 170061P 1 NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised Commissioning Nursing Trans. & Corp. Ops. Commissioning Strategy Finance Publications Gateway Reference: 07603 Document Purpose Policy Clinical commissioning policy: Susoctocog alfa for treating bleeding Document Name episodes in people with acquired haemophilia A (all ages) Author Specialised Commissioning Team Publication Date 29 June 2018 Target Audience CCG Clinical Leaders, Care Trust CEs, Foundation Trust CEs , Medical Directors, Directors of PH, Directors of Nursing, NHS England Regional Directors, NHS England Directors of Commissioning Operations, Directors of Finance, NHS Trust CEs Additional Circulation #VALUE! List Description Routinely Commissioned - NHS England will routinely commission this specialised treatment in accordance with the criteria described in this policy. Cross Reference 0 Superseded Docs 0 (if applicable) Action Required 0 Timing / Deadlines By 00 January 1900 (if applicable) Contact Details for [email protected] further information 0 0 0 0 0 0 Document Status This is a controlled document. Whilst this document may be printed, the electronic version posted on the intranet is the controlled copy. Any printed copies of this document are not controlled. As a controlled document, this document should not be saved onto local or network drives but should always be accessed from the intranet. 2 Standard Operating Procedure: Clinical Commissioning Policy: Susoctocog alfa for treating bleeding episodes in people with acquired haemophilia A (all ages) First published: June 2018 Prepared by the National Institute for Health and Care Excellence (NICE) Commissioning Support Programme Published by NHS England, in electronic format only. -

Late Stent Thrombosis After Paclitaxel-Eluting Stent Placement in a Patient with Essential Thrombocytosis
558 Türk Kardiyol Dern Arş - Arch Turk Soc Cardiol 2010;38(8):558-560 Late stent thrombosis after paclitaxel-eluting stent placement in a patient with essential thrombocytosis Esansiyel trombositozlu bir hastada paklitaksel salınımlı stent yerleştirme sonrası gelişen geç stent trombozu Telat Keleş, M.D., Nihal Akar Bayram, M.D., Tahir Durmaz, M.D., Engin Bozkurt, M.D. Department of Cardiology, Ankara Atatürk Education and Research Hospital, Ankara We report on a case of late stent thrombosis after drug- Bu yazıda, esansiyel trombositozlu bir hastada ilaç sa- eluting stent placement in a patient with essential throm- lınımlı stent yerleştirme sonrası gelişen geç stent trom- bocytosis. A 51-year-old male patient with a three-month bozu sunuldu. Üç ay önce sol ön inen artere paklitak- history of paclitaxel-eluting stent placement to the left sel salınımlı stent yerleştirilen 51 yaşındaki erkek hasta anterior descending artery presented with a complaint şiddetli retrosternal göğüs ağrısı yakınmasıyla başvur- of severe retrosternal chest pain. A high platelet count du. İki ay öncesinde hastanın trombosit sayımı yüksek (1,063,000/mm3) was detected two months prior to (1063000/mm3) bulunmuş ve durumu esansiyel trombo- presentation, which was interpreted as essential throm- sitoz olarak yorumlanmıştı. Hasta standart ikili antitrom- bocytosis. He was on standard dual antiplatelet therapy bosit tedavi (aspirin ve klopidogrel) görmekteydi. Elekt- (aspirin and clopidogrel). The electrocardiogram showed rokardiyografide V1-V6 derivasyonlarında ST-segment ST-segment elevation in leads V1-V6. Emergent coro- yükselmesi izlendi. Acil koroner anjiyografide paklitaksel nary angiography revealed thrombotic total occlusion salınımlı stent yerinde trombotik tam tıkanıklık gözlendi. at the location of the paclitaxel-eluting stent. -
Coagulation Factor IX (Recombinant) - Drugbank
12/7/2017 Coagulation Factor IX (Recombinant) - DrugBank Coagulation Factor IX (Recombinant) Targets (7) Biointeractions (4) IDENTIFICATION Name Coagulation Factor IX (Recombinant) Accession Number DB00100 (BTD00038, BIOD00038) Type Biotech Groups Approved Biologic Classification Protein Based Therapies Blood factors Description Recombinant Coagulation Factor IX is a purified Factor IX glycoprotein produced by recombinant DNA technology. It has a primary amino acid sequence that is identical to the Ala148 allelic form of human factor IX, and has structural and functional characteristics similar to those of endogenous factor IX. It is not derived from human blood (unlike human Factor IX complex), and is instead produced by a genetically engineered Chinese hamster ovary (CHO) cell line that secretes recombinant Factor IX into cell medium that is then processed and purified for use as a pharmaceutical agent. Recombinant Factor IX is indicated for the control and prevention of bleeding episodes in adult and pediatric patients with congenital factor IX deficiency (Hemophilia B). Protein structure Protein chemical formula C2041H3136N558O641S25 Protein average weight 46548.2 Da https://www.drugbank.ca/drugs/DB00100 1/13 12/7/2017 Coagulation Factor IX (Recombinant) - DrugBank 46548.2 Da Sequences >DB00100 sequence YNSGKLEEFVQGNLERECMEEKCSFEEAREVFENTERTTEFWKQYVDGDQCESNPCLNGG SCKDDINSYECWCPFGFEGKNCELDVTCNIKNGRCEQFCKNSADNKVVCSCTEGYRLAEN QKSCEPAVPFPCGRVSVSQTSKLTRAEAVFPDVDYVNSTEAETILDNITQSTQSFNDFTR VVGGEDAKPGQFPWQVVLNGKVDAFCGGSIVNEKWIVTAAHCVETGVKITVVAGEHNIEE -

May 2020 for Health Professionals Who Care for Cancer Patients
Systemic Therapy Update Volume 23 Issue 5 May 2020 For Health Professionals Who Care for Cancer Patients Inside This Issue: Editor’s Choice Benefit Drug List New Programs: Neoadjuvant Carboplatin for Triple-Negative New: BRLACTWAC, UGUPAPA, Pegaspargase (LKNOS, Breast Cancer (BRLACTWAC) | Apalutamide for Non- LYNOS), Bevacizumab (Pediatric), IGEV (Pediatric) | Metastatic Castration-Resistant Prostate Cancer (UGUPAPA) Deleted: GIGAVEOCAP, GIGAVEOF, SMAJIFN Drug Update NEW Protocols, PPPOs and Patient Handouts Pharmacare Update – Anticoagulants | Patient Assistance BR: BRLACTWAC | GU: UGUPAPA Programs REVISED Protocols, PPPOs and Patient Handouts Drug Shortages BR: BRAJAC, BRAJACT, BRAJACTG, BRAJACTT, BRAJACTTG, New: Alemtuzumab, Anagrelide | Updated: Hydroxyurea BRAJACTW, BRAJDAC, BRAJFEC, BRAJFECD, BRAJFECDT, Cancer Drug Manual© BRAVAC, BRLAACD, BRLAACDT, BRLATACG, BRLATWAC | New: Abemaciclib, Lurbinectedin | Revised: Apalutamide, GI: GIPGEMABR | GU: UGUPABI, UGUPENZ | LK: ULKBLIN, Durvalumab, Panitumumab, Pembrolizumab ULKMDSA | SA: SAIME | SC: SCDRUGRX | SM: USMAVDAB, ST Update Editorial Board USMAVDT, USMAVTRA, USMAVVC, USMAVVEM Membership Update Resources and Contact Information Editor’s Choice New Programs Effective 01 May 2020, the BC Cancer Provincial Systemic Therapy Program has approved the following new treatment programs. The full details of these programs can be found on the BC Cancer website in the Chemotherapy Protocols section. Breast Neoadjuvant Carboplatin for Locally Advanced, Triple-Negative Breast Cancer (BRLACTWAC) — The Provincial Systemic Therapy Program has approved the addition of carboplatin to the standard taxane- anthracycline neoadjuvant chemotherapy regimens, for patients with stage II and higher triple-negative breast cancer. This regimen consists of carboplatin (given every 3 weeks) in combination with weekly paclitaxel, followed by doxorubicin-cyclophosphamide (AC given every 2 or 3 weeks). Filgrastim support is available for the dose-dense AC option. -

081999 Disseminated Intravascular Coagulation
The New England Journal of Medicine Current Concepts Systemic activation+ of coagulation DISSEMINATED INTRAVASCULAR COAGULATION Intravascular+ Depletion of platelets+ deposition of fibrin and coagulation factors MARCEL LEVI, M.D., AND HUGO TEN CATE, M.D. Thrombosis of small+ Bleeding and midsize vessels+ ISSEMINATED intravascular coagulation is and organ failure characterized by the widespread activation Dof coagulation, which results in the intravas- Figure 1. The Mechanism of Disseminated Intravascular Coag- cular formation of fibrin and ultimately thrombotic ulation. occlusion of small and midsize vessels.1-3 Intravascu- Systemic activation of coagulation leads to widespread intra- lar coagulation can also compromise the blood sup- vascular deposition of fibrin and depletion of platelets and co- agulation factors. As a result, thrombosis of small and midsize ply to organs and, in conjunction with hemodynam- vessels may occur, contributing to organ failure, and there may ic and metabolic derangements, may contribute to be severe bleeding. the failure of multiple organs. At the same time, the use and subsequent depletion of platelets and coag- ulation proteins resulting from the ongoing coagu- lation may induce severe bleeding (Fig. 1). Bleeding may be the presenting symptom in a patient with disseminated intravascular coagulation, a factor that can complicate decisions about treatment. TABLE 1. COMMON CLINICAL CONDITIONS ASSOCIATED WITH DISSEMINATED ASSOCIATED CLINICAL CONDITIONS INTRAVASCULAR COAGULATION. AND INCIDENCE Sepsis Infectious Disease Trauma Serious tissue injury Disseminated intravascular coagulation is an ac- Head injury Fat embolism quired disorder that occurs in a wide variety of clin- Cancer ical conditions, the most important of which are listed Myeloproliferative diseases in Table 1. -

What Everyone Should Know to Stop Bleeding After an Injury
What Everyone Should Know to Stop Bleeding After an Injury THE HARTFORD CONSENSUS The Joint Committee to Increase Survival from Active Shooter and Intentional Mass Casualty Events was convened by the American College of Surgeons in response to the growing number and severity of these events. The committee met in Hartford Connecticut and has produced a number of documents with rec- ommendations. The documents represent the consensus opinion of a multi-dis- ciplinary committee involving medical groups, the military, the National Security Council, Homeland Security, the FBI, law enforcement, fire rescue, and EMS. These recommendations have become known as the Hartford Consensus. The overarching principle of the Hartford Consensus is that no one should die from uncontrolled bleeding. The Hartford Consensus recommends that all citizens learn to stop bleeding. Further information about the Hartford Consensus and bleeding control can be found on the website: Bleedingcontrol.org 2 SAVE A LIFE: What Everyone Should Know to Stop Bleeding After an Injury Authors: Peter T. Pons, MD, FACEP Lenworth Jacobs, MD, MPH, FACS Acknowledgements: The authors acknowledge the contributions of Michael Cohen and James “Brooks” Hart, CMI to the design of this manual. Some images adapted from Adam Wehrle, EMT-P and NAEMT. © 2017 American College of Surgeons CONTENTS SECTION 1 3 ■ Introduction ■ Primary Principles of Trauma Care Response ■ The ABCs of Bleeding SECTION 2 5 ■ Ensure Your Own Safety SECTION 3 6 ■ A – Alert – call 9-1-1 SECTION 4 7 ■ B – Bleeding – find the bleeding injury SECTION 5 9 ■ C – Compress – apply pressure to stop the bleeding by: ■ Covering the wound with a clean cloth and applying pressure by pushing directly on it with both hands, OR ■Using a tourniquet, OR ■ Packing (stuff) the wound with gauze or a clean cloth and then applying pressure with both hands SECTION 6 13 ■ Summary 2 SECTION 1: INTRODUCTION Welcome to the Stop the Bleed: Bleeding Control for the Injured information booklet. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -
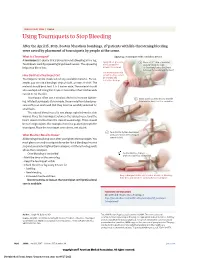
Using Tourniquets to Stop Bleeding
JAMA PATIENT PAGE | Trauma Using Tourniquets to Stop Bleeding After the April 15, 2013, Boston Marathon bombings, 27 patients with life-threatening bleeding were saved by placement of tourniquets by people at the scene. What Is a Tourniquet? Applying a tourniquet with a windlass device A tourniquet is a device that is placed around a bleeding arm or leg. Apply direct pressure 1 Place a 2-3” strip of material Tourniquets work by squeezing large blood vessels. The squeezing to the wound for about 2” from the edge helps stop blood loss. at least 15 minutes. of the wound over a long bone between the wound and the heart. Use a tourniquet only How Do I Put a Tourniquet On? when bleeding cannot be stopped and Tourniquets can be made out of any available material. For ex- is life threatening. ample, you can use a bandage, strip of cloth, or even a t-shirt. The material should be at least 2 to 3 inches wide. The material should also overlap itself. Using thin straps or material less than 2 inches wide can rip or cut the skin. Tourniquets often use a windlass device to increase tighten- 2 Insert a stick or other strong, straight ing. Inflated tourniquets (for example, those made from blood pres- item into the knot to act as a windlass. sure cuffs) can work well. But they must be carefully watched for small leaks. The injured blood vessel is not always right below the skin wound. Place the tourniquet between the injured vessel and the heart, about 2 inches from the closest wound edge. -

A Guide for People Living with Von Willebrand Disorder CONTENTS
A guide for people living with von Willebrand disorder CONTENTS What is von Willebrand disorder (VWD)?................................... 3 Symptoms............................................................................................... 5 Types of VWD...................................................................................... 6 How do you get VWD?...................................................................... 7 VWD and blood clotting.................................................................... 11 Diagnosis................................................................................................. 13 Treatment............................................................................................... 15 Taking care of yourself or your child.............................................. 19 (Education, information, first aid/medical emergencies, medication to avoid) Living well with VWD......................................................................... 26 (Sport, travel, school, telling others, work) Special issues for women and girls.................................................. 33 Connecting with others..................................................................... 36 Can I live a normal life with von Willebrand disorder?............. 37 More information................................................................................. 38 2 WHAT IS VON WILLEBRAND DISORDER (VWD)? Von Willebrand disorder (VWD) is an inherited bleeding disorder. People with VWD have a problem with a protein -

Xagrid, INN-Anagrelide
SCIENTIFIC DISCUSSION 1. Introduction Anagrelide hydrochloride monohydrate is a novel imidazoquinazoline originally developed as an inhibitor of platelet aggregation but subsequently found to have value as a platelet-lowering agent for the treatment of patients suffering from Essential thrombocythaemia (ET). ET is one of a number of chronic myeloproliferative disorders characterised by an elevated platelet count due to an autonomous clonal proliferation of bone marrow megakaryocytes. The underlying cause of this proliferation is unknown. Platelet function is generally normal and the cells are able to undergo aggregation and to participate in the haemostatic process in a more or less normal manner. The diagnosis is one of exclusion of reactive causes and other myeloproliferative disorders (MPDs) according to internationally agreed criteria of the Polycythaemia Vera Study Group (PVSG). The main criterion is a sustained platelet level of >600 x 109/L for more than two months. It is estimated that the prevalence of ET in the European Union (EU) is 2-3 per 10,000 persons. ET is a condition that may affect anyone at any stage of life from childhood although the median age of presentation is 60 years. Literature and clinical experience indicate a preponderance of female patients between the ages of 25 and 50 years. Up to two thirds of patients with ET are asymptomatic and a significant number of patients are now being diagnosed as an incidental consequence of the analysis of full blood counts for other health reasons. Generally, the older patient is more likely to be symptomatic at presentation, although younger patients may present with major complications. -

Justification
Justification to the Resolution of the Federal Joint Committee (G-BA) on an Amendment of the Pharmaceuticals Directive (AM ‑ RL): Annex XII – Resolutions on the Benefit Assessment of Medicinal Products with New Active Ingredients in Accordance with Section 35a SGB V Damoctocog alfa pegol From 20 June 2019 Contents 1. Legal basis ................................................................................................................ 2 2. Key points of the resolution ..................................................................................... 2 2.1 Additional benefit of the medicinal product in relation to the appropriate comparator therapy ..................................................................................................... 3 2.1.1 Approved therapeutic indication of damoctocog alfa pegol (Jivi®) in accordance with the summary of product characteristics ............................................ 3 2.1.2 Appropriate comparator therapy ................................................................... 3 2.1.3 Extent and probability of the additional benefit .............................................. 5 2.1.4 Summary of the assessment ........................................................................ 6 2.2 Number of patients or demarcation of patient groups eligible for treatment ...... 6 2.3 Requirements for a quality-assured application ................................................ 7 2.4 Treatment costs ..............................................................................................