Evolution of Stickleback in 50 Years on Earthquake-Uplifted Islands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SPYCATCHER by PETER WRIGHT with Paul Greengrass WILLIAM
SPYCATCHER by PETER WRIGHT with Paul Greengrass WILLIAM HEINEMANN: AUSTRALIA First published in 1987 by HEINEMANN PUBLISHERS AUSTRALIA (A division of Octopus Publishing Group/Australia Pty Ltd) 85 Abinger Street, Richmond, Victoria, 3121. Copyright (c) 1987 by Peter Wright ISBN 0-85561-166-9 All Rights Reserved. No part of this publication may be reproduced, stored in or introduced into a retrieval system, or transmitted, in any form or by any means (electronic, mechanical, photocopying, recording or otherwise) without the prior written permission of the publisher. TO MY WIFE LOIS Prologue For years I had wondered what the last day would be like. In January 1976 after two decades in the top echelons of the British Security Service, MI5, it was time to rejoin the real world. I emerged for the final time from Euston Road tube station. The winter sun shone brightly as I made my way down Gower Street toward Trafalgar Square. Fifty yards on I turned into the unmarked entrance to an anonymous office block. Tucked between an art college and a hospital stood the unlikely headquarters of British Counterespionage. I showed my pass to the policeman standing discreetly in the reception alcove and took one of the specially programmed lifts which carry senior officers to the sixth-floor inner sanctum. I walked silently down the corridor to my room next to the Director-General's suite. The offices were quiet. Far below I could hear the rumble of tube trains carrying commuters to the West End. I unlocked my door. In front of me stood the essential tools of the intelligence officer’s trade - a desk, two telephones, one scrambled for outside calls, and to one side a large green metal safe with an oversized combination lock on the front. -

The Evolution of British Intelligence Assessment, 1940-41
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies Legacy Theses 1999 The evolution of British intelligence assessment, 1940-41 Tang, Godfrey K. Tang, G. K. (1999). The evolution of British intelligence assessment, 1940-41 (Unpublished master's thesis). University of Calgary, Calgary, AB. doi:10.11575/PRISM/18755 http://hdl.handle.net/1880/25336 master thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca THE UNIVERSITY OF CALGARY The Evolution of British Intelligence Assessment, 1940-41 by Godfiey K. Tang A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FLTLFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF ARTS DEPARTMENT OF HISTORY CALGARY, ALBERTA JANUARY, 1999 O Godfiey K Tang 1999 National Library Biblioth4que nationale #*lof Canada du Canada Acquisitions and Acquisitions et Bibliographic Services services bibliographiques 395 Wellington Street 395, rue Wellington OnawaON K1AON4 Ottawa ON KIA ON4 Canada Canada The author has granted a non- L'auteur a accorde une licence non exclusive licence allowing the exclusive pernettant Pla National Library of Canada to Blbliotheque nationale du Canada de reproduce, loan, distribute or sell reproduire, preter, distciiuer ou copies of this thesis in microform, vendre des copies de cette these sous paper or electronic formats. la forme de microfiche/f&n, de reproduction sur papier ou sur format Bectronique. -

A Visual Motion Detection Circuit Suggested by Drosophila Connectomics
ARTICLE doi:10.1038/nature12450 A visual motion detection circuit suggested by Drosophila connectomics Shin-ya Takemura1, Arjun Bharioke1, Zhiyuan Lu1,2, Aljoscha Nern1, Shiv Vitaladevuni1, Patricia K. Rivlin1, William T. Katz1, Donald J. Olbris1, Stephen M. Plaza1, Philip Winston1, Ting Zhao1, Jane Anne Horne2, Richard D. Fetter1, Satoko Takemura1, Katerina Blazek1, Lei-Ann Chang1, Omotara Ogundeyi1, Mathew A. Saunders1, Victor Shapiro1, Christopher Sigmund1, Gerald M. Rubin1, Louis K. Scheffer1, Ian A. Meinertzhagen1,2 & Dmitri B. Chklovskii1 Animal behaviour arises from computations in neuronal circuits, but our understanding of these computations has been frustrated by the lack of detailed synaptic connection maps, or connectomes. For example, despite intensive investigations over half a century, the neuronal implementation of local motion detection in the insect visual system remains elusive. Here we develop a semi-automated pipeline using electron microscopy to reconstruct a connectome, containing 379 neurons and 8,637 chemical synaptic contacts, within the Drosophila optic medulla. By matching reconstructed neurons to examples from light microscopy, we assigned neurons to cell types and assembled a connectome of the repeating module of the medulla. Within this module, we identified cell types constituting a motion detection circuit, and showed that the connections onto individual motion-sensitive neurons in this circuit were consistent with their direction selectivity. Our results identify cellular targets for future functional investigations, and demonstrate that connectomes can provide key insights into neuronal computations. Vision in insects has been subject to intense behavioural1,physiological2 neuroanatomy14. Given the time-consuming nature of such recon- and anatomical3 investigations, yet our understanding of its underlying structions, we wanted to determine the smallest medulla volume, neural computations is still far from complete. -

A Pilot Trap Survey of Artificial Reefs in New Jersey for Monitoring of Black Sea Bass, Tautog, and Lobster
Final Report Submitted to the New Jersey Department of Environmental Protection, Division of Fish and Wildlife for the following project: Project Title: A Pilot Trap Survey of Artificial Reefs in New Jersey for Monitoring of Black Sea Bass, Tautog, and Lobster Organization Name: Rutgers, the State University of New Jersey Principal Investigator: Dr. Olaf P. Jensen, Associate Professor, Rutgers University ([email protected], 410-812-4842) Project Co-Investigator: Dr. Douglas Zemeckis, Postdoctoral Researcher, Rutgers University ([email protected], 848-932-3450) NJDEP Project Manager: Peter Clarke, Fisheries Biologist, NJDEP Division of Fish and Wildlife ([email protected]) Performance Period: January 1, 2016 through April 1, 2019 Total Budget: $201,905.00 Table of Contents Introduction……………………………………………………………………………………...1 Project Objectives……………………………………………………………………………….6 Methods………………………………………………………………………………………......8 Study Design: Locations and Times……………………………………………………....8 Protocol: Field and Laboratory Methods…………………………………………………10 2016 Spring Seasonal Monitoring of Artificial Reefs…………………………………….11 2016 Summer Seasonal Monitoring of Artificial Reefs…………………………………..12 2016 Fall Seasonal Monitoring of Artificial Reefs………………………………………13 2017 Spring Seasonal Monitoring of Artificial Reefs…………………………………….14 2017 Summer Seasonal Monitoring of Artificial Reefs……………………………..……15 2017 Fall Seasonal Monitoring of Artificial Reefs………………………………………17 2018 Spring Seasonal Monitoring of Artificial Reefs……………………………………18 2018 Summer Seasonal -

Monday/Tuesday Playoff Schedule
2013 TUC MONDAY/TUESDAY PLAYOFF MASTER FIELD SCHEDULE Start End Hockey1 Hockey2 Hockey3 Hockey4 Hockey5 Ulti A Soccer 3A Soccer 3B Cricket E1 Cricket E2 Cricket N1 Cricket N2 Field X 8:00 9:15 MI13 MI14 TI13 TI14 TI15 TI16 MI1 MI2 MI3 MI4 MI15 MI16 9:20 10:35 MI17 MI18 TI17 TI18 TI19 TI20 MI5 MI6 10:40 11:55 MI19 MI20 MC1 MC2 MC3 MI21 MI7 MI8 12:00 1:15 MI9* TI21* TI22 TI23 TI24 MI10 MI11 MI12 1:20 2:35 MI22 MC4 MC6 MC5 MI23 TC1 MI24 MI25 2:40 3:55 TI1 TI2 MC7 TI3 MI26 TC2 TR1 TR2 MI27 4:00 5:15 MC8* TC3 MC10 MC9 TI4 TC4 TR3 TR4 5:20 6:35 TC5* TI5 TI6 TI7 TI8 TC6 TR5 TR6 6:40 7:55 TI9* TC7 TI10 TI11 TI12 TC8 TR8 TR7 Games are to 15 points Half time at 8 points Games are 1 hour and 15 minutes long Soft cap is 10 minutes before the end of game, +1 to highest score 2 Timeouts per team, per game NO TIMEOUTS AFTER SOFT CAP Footblocks not allowed, unless captains agree otherwise 2013 TUC Monday Competitive Playoffs - 1st to 7th Place 3rd Place Bracket Loser of MC4 Competitive Teams Winner of MC9 MC9 Allth Darth (1) Allth Darth (1) 3rd Place Slam Dunks (2) Loser of MC5 The Ligers (3) Winner of MC4 MC4 Krash Kart (4) Krash Kart (4) The El Guapo Sausage Party (5) MC1 Wonky Pooh (6) Winner of MC1 Disc Horde (7) The El Guapo Sausage Winner of MC8 Party (5) MC8 Slam Dunks (2) Champions Winner of MC2 MC2 Disc Horde (7) MC5 The Ligers (3) Winner of MC5 MC3 Winner of MC3 Wonky Pooh (6) Time Hockey3 Score Spirit Hockey4 Score Spirit Hockey5 Score Spirit Score Spirit 10:40 Krash Kart (4) Slam Dunks (2) The Ligers (3) to vs. -

Confidence Men the Mediterranean Double-Cross System, 1941-45 By
Confidence Men The Mediterranean Double-Cross System, 1941-45 by Brett Edward Lintott A thesis submitted in conformity with the requirements for the Degree of Doctor of Philosophy, Graduate Department of History, in the University of Toronto © Copyright by Brett Edward Lintott, 2015 Abstract Confidence Men The Mediterranean Double-Cross System, 1941-45 Brett Edward Lintott Doctor of Philosophy Department of History University of Toronto, 2015 This dissertation provides an analysis of the Mediterranean double-cross system of the Second World War, which was composed of a number of double agents who were turned by the Allies and operated against their ostensible German spymasters. Utilizing many freshly released archival materials, this study assesses how the double-cross system was constructed, why it was an effective instrument, and how it contributed to Allied success in two areas: security and counter-intelligence, and military deception. The focus is thus on both organization and operations. The chapters cover three chronological periods. In the first — 1941-42 — the initial operational usage of a double agent is assessed, along with the development of early organizational structures to manage and operate individual cases as components of a team of spies. The second section, covering 1943, assesses three issues: major organizational innovations made early that year; the subsequent use of the double agent system to deceive the Germans regarding the planned invasion of Sicily in July; and the ongoing effort to utilize double agents to ensure a stable security and counter-intelligence environment in the Mediterranean theatre. The third and final section analyzes events in 1944, with a focus on double-cross deception in Italy and France, and on the emergence of more systematic security and counter-intelligence double-cross operations in Italy and the Middle East. -

Here the Policymaking Value of Fresh Thinking and Cognitive Diversity Combined with Seasoned Expertise and Accumulated Wisdom Has Long Been Recognised
GLOBAL STRATEGY FORUM Lecture Series 2018 - 2019 www.globalstrategyforum.org Lord Lothian, Mr. Radek Sikorski and Sir Malcolm Rifkind Sir John Chilcot and Lord Lothian Mr. James Barr and Lord Lothian Professor Charles Garraway and Lord Lothian Lord Lothian and Mr. Ben Macintyre Dr. Kori Schake and Lord Lothian Mr. Matthew Rycroft and Lord Lothian Lord Lothian and Mr. Gordon Corera www.globalstrategyforum.org GLOBAL STRATEGY FORUM Lecture Series 2018 - 2019 3 www.globalstrategyforum.org NOTES 4 www.globalstrategyforum.org PRESIDENT’S FOREWORD It gives me great pleasure to introduce this, the thirteenth edition of GSF’s annual lecture publication. In these pages you will once again find a full record of the extensive events programme which we delivered during the course of our 2018-2019 series. Topics and regions predictably included Brexit, China, Russia, the Middle East and the US, as well the big global issues of the day: climate change, terrorism, globalisation, cybersecurity. But the breadth and range of countries, region and topics covered was striking, from Brazil to Yemen, and from international development and the Commonwealth to the return of great power rivalry, attracting record audiences along the way. Unsurprisingly, much focus and political capital has continued to lie with the Brexit process, which has dominated the public discourse. But in GSF debates throughout the year on the UK’s role in the world, I observed a clear desire – demand, even - for substance to be given to the concept of ‘Global Britain’ and a firm eschewal of any reduction in our engagement in world events. During this period of change and uncertainty in the UK and beyond, the answers to the many complicated questions of policy and strategy facing us remain elusive, but GSF’s mandate requires us to continue to strive to seek them. -

2014 Tuc Monday/Tuesday Playoff Master Field Schedule
2014 TUC MONDAY/TUESDAY PLAYOFF MASTER FIELD SCHEDULE Start End Hockey 1 Hockey 2 Hockey 3 Hockey 4 Hockey 5 Hockey 6 Ulti A Rugby E1 Rugby E2 9:20 10:35 MI1 MI2 MC1 MC2 MI8 MI9 10:40 11:55 MI3 MI4 MC5 MC3 MC4 MI11 MI5 MI10 12:00 1:15 MI6 MI7 MC7 1:30 2:55 ASI1 ASI2 ASC1 ASC2 MC6 3:00 4:25 ASI3 ASI4 ASC4 ASC3 4:30 5:45 T5 T6 T2 T1 5:50 7:05 T7 T8 T4 T3 TOURNAMENT RULES Games are to 15 points Half time at 8 points Playoff games are 1 hour and 15 minutes long Hard cap is at 75 minutes (finish the point, only if tied do you play another), and there is no soft cap. 1 Timeout per half, per team (no timeouts if in overtime) Footblocks are not allowed, unless captains agree otherwise 2014 TUC Monday Competitive Playoffs - 1st to 6th Place Teams 3rd Place Deep (1) Deep (1) L - MC3 Allth Darth (2) 3rd Place MC7 naptime (3) W - MC3 MC3 Slam Dunks (4) Slam Dunks (4) L - MC4 El Guapo (5) MC1 Basic Bishes (6) W - MC1 5th Place El Guapo (5) Champions L - MC1 MC6 5th Place MC5 Allth Darth (2) L - MC2 MC4 naptime (3) W - MC4 MC2 W - MC2 Basic Bishes (6) Time Hockey 4 Score Spirit Hockey 6 Score Spirit 9:20 Slam Dunks (4) naptime (3) to vs. MC 1 vs. MC 2 10:35 El Guapo (5) Basic Bishes (6) Time Hockey 4 Score Spirit Hockey 6 Score Spirit Hockey 3 Score Spirit 10:40 Deep (1) Allth Darth (2) L - MC1 to vs. -
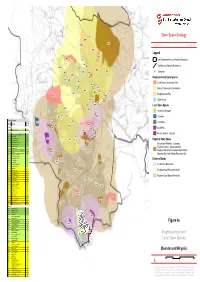
7929 GIS 104 Fig 4A Neighbourhood and Local Open Space
ML1 Open Space Strategy MR1 MF1 MR10 January 2014 7929 GIS 104 MR1 Legend MP2 MS3 MI6 East Dunbartonshire Local Authority Boundary MI7 MI5 Scottish Local Authority Boundaries MS4 MI9 BLF1 MR5 MI15 ! MP3 MI2 MR1 Allotments MR4 MI12 Neighbourhood Open Spaces MS7 MI4 MI1 Local Nature Conservation Site MI10 MI13 MI11 MI3 Natural / Semi-natural Greenspace MR6 MS6 Neighbourhood Park MS2 MR8 MI14 Sports Areas ML2 MR9 MR3 Local Open Spaces BI18 MI8 BI8 Amenity Greenspace BI1 BI2 BI15 MR7 Cemetery BI16 Fitness for Purpose BR6 Quality BF2 Civic Space Site Ref. Site Name MR2 Score BR5 BI14 Baldernock BI4 Local Park BLF1 Baldernock Cemetery 69 BR12 Bearsden BI6 BR2 Kilmardinny Loch Local Nature Reserve 85 Private Gardens / Grounds BR12 Mosshead Park 81 BI11 BP2 Glasgow Uni. Woods 79 BR3 BI10 Regional Open Space BR3 Langfaulds Field 78 BR2 BI17 Bearsden War Memorial 76 BR4 King George V Park 76 Recreational Walkway / Cycleway; BS2 Cairnhill Woods 76 Regional Historic / Natural Attraction; BR1 Colquhoun Park 74 BI4 Grampian Way and Cruchan Road O.S. 73 BI13 Regional Site of Nature Conservation Interest; BR7 Thorn Park 70 BR11 BR6 Heather Ave Open Space 68 Regional Sport and Outdoor Recreation Site BF1 New Kilpatrick Cemetery 67 BI15 Paterson Place O.S. 66 BR8 BS1 Templehill Woods 65 BF1 Distance Bands BR11 Antonine Park 65 BI12 BI18 Stockie Muir Road OS 2 65 Local Parks 400m buffer BI5 Braemar Cres. O.S. 65 BR8 Roman Park 64 BS3 Cairnhill Woods 64 BI17 Neighbourhood Parks 840m buffer BF2 Langfaulds Cemetery 63 BR7 BI2 Stockie Muir Road 1 63 BI16 Abercrombie Dr. -

Nigel West, 2009
OTHER A TO Z GUIDES FROM THE SCARECROW PRESS, INC. 1. The A to Z of Buddhism by Charles S. Prebish, 2001. 2. The A to Z of Catholicism by William J. Collinge, 2001. 3. The A to Z of Hinduism by Bruce M. Sullivan, 2001. 4. The A to Z of Islam by Ludwig W. Adamec, 2002. 5. The A to Z of Slavery & Abolition by Martin A. Klein, 2002. 6. Terrorism: Assassins to Zealots by Sean Kendall Anderson and Stephen Sloan, 2003. 7. The A to Z of the Korean War by Paul M. Edwards, 2005. 8. The A to Z of the Cold War by Joseph Smith and Simon Davis, 2005. 9. The A to Z of the Vietnam War by Edwin E. Moise, 2005. 10. The A to Z of Science Fiction Literature by Brian Stableford, 2005. 11. The A to Z of the Holocaust by Jack R. Fischel, 2005. 12. The A to Z of Washington, D.C. by Robert Benedetto, Jane Dono- van, and Kathleen DuVall, 2005. 13. The A to Z of Taoism by Julian F. Pas, 2006. 14. The A to Z of the Renaissance by Charles G. Nauert, 2006. 15. The A to Z of Shinto by Stuart D. B. Picken, 2006. 16. The A to Z of Byzantium by John H. Rosser, 2006. 17. The A to Z of the Civil War by Terry L. Jones, 2006. 18. The A to Z of the Friends (Quakers) by Margery Post Abbott, Mary Ellen Chijioke, Pink Dandelion, and John William Oliver Jr., 2006 19. -

The Detention of Non-Enemy Civilians Escaping to Britain During the Second World War
The Historical Journal (2021), 1–23 doi:10.1017/S0018246X2100008X ARTICLE The Detention of Non-Enemy Civilians Escaping to Britain during the Second World War Artemis J. Photiadou Department of International History, London School of Economics and Political Science, London, UK E-mail: [email protected] Abstract Thousands of civilians from Allied and neutral countries reached Britain during the Second World War. Nearly all who arrived between 1941 and 1945 were detained for interrogation – an unprecedented course of action by Britain which has nevertheless seldomly been studied. This article focuses on the administrative history of this process and the people it affected. It demonstrates how certain parts of the state treated non- Britons with suspicion throughout the war, long after fears of a ‘fifth column’ had sub- sided. At the same time, others saw them favourably, not least because many either offered intelligence, intended to volunteer with the Allied Forces, or work for the war industry. Examining how these conflicting views co-existed within a single deten- tion camp, this article thus illustrates the complex relationship that existed between non-Britons and the wartime state, which perceived them simultaneously as suspects, assets, and allies. By making use of the thousands of resulting interrogation reports, the article also offers more detail than currently exists on the gender and nationality background of those who reached Britain, as well as about the journeys they took to escape occupied territory. Chaim Wasserman, a young Jewish man from Poland, was in a detention camp in London when the war in Europe ended. The camp authorities described him as a student, but that had been his status six years earlier, when at the age of eighteen he was sent to live in a ghetto in Warsaw, then to several prison camps, then to Auschwitz, and eventually to Buchenwald, from where he escaped in April 1945. -

The Secret Listeners
The Secret Listeners The Secret Listeners Pascal Theatre Company The Secret Listeners Edited by Julia Pascal and Thomas Kampe Pascal Theatre Company ‘The project set out to investigate little Edited by Julia Pascal and Thomas Kampe known events that happened during World War II at the large mansion house in Trent Park ..... What was the particular history that had been hidden for so long?’ ‘A site-specific investigation, a series of interviews with refugees and locals, a learning process and training for volunteers, a film, a public discussion, a new research document, a website, a book and much more.’ Julia Pascal ‘The performance event central to this heritage educational project was conceived as a site-responsive tour, as a promenade-like immersive experience for the spectators as active, emancipated participants.’ Thomas Kampe The Secret Listeners Pascal Theatre Company Edited by Julia Pascal and Thomas Kampe A Brief History 7 Julia Pascal From Black Box to Open House 11 What is Political Theatre? Julia Pascal Listening as Learning 21 Thomas Kampe An Emerging Vision: Secret Listeners 23 Working Methods Thomas Kampe Partnerships 41 The Wiener Library and The Jewish Military Museum Research and Future Questions 45 Sally Mijit ATrentParkHistory 67 Melvyn Keen Reflections 73 Jonathan Meth, Mark Norfolk, Del Taylor, Susannah Kraft Levene, Wayne McGee and Lesley Lightfoot Credits 86 Julia Pascal ABriefHistory This book gives an insight into a heritage arts educational project undertaken in 2012-2013 by Pascal Theatre Company. The Secret Listeners was made possible by a grant from the Heritage Lottery Fund (HLF). The project set out to investigate little known events that happened during World War II at the large mansion house in Trent Park, and from that exploration, several strands of educational work, which ran parallel to different activities during 2012 and 2013.