Loss of DACH2 Is a Candidate Early Event in Breast and Ovarian Carcinogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Análise Integrativa De Perfis Transcricionais De Pacientes Com
UNIVERSIDADE DE SÃO PAULO FACULDADE DE MEDICINA DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM GENÉTICA ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas Ribeirão Preto – 2012 ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas Tese apresentada à Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo para obtenção do título de Doutor em Ciências. Área de Concentração: Genética Orientador: Prof. Dr. Eduardo Antonio Donadi Co-orientador: Prof. Dr. Geraldo A. S. Passos Ribeirão Preto – 2012 AUTORIZO A REPRODUÇÃO E DIVULGAÇÃO TOTAL OU PARCIAL DESTE TRABALHO, POR QUALQUER MEIO CONVENCIONAL OU ELETRÔNICO, PARA FINS DE ESTUDO E PESQUISA, DESDE QUE CITADA A FONTE. FICHA CATALOGRÁFICA Evangelista, Adriane Feijó Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas. Ribeirão Preto, 2012 192p. Tese de Doutorado apresentada à Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo. Área de Concentração: Genética. Orientador: Donadi, Eduardo Antonio Co-orientador: Passos, Geraldo A. 1. Expressão gênica – microarrays 2. Análise bioinformática por module maps 3. Diabetes mellitus tipo 1 4. Diabetes mellitus tipo 2 5. Diabetes mellitus gestacional FOLHA DE APROVAÇÃO ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas. -

Comparative Transcriptomics Reveals Similarities and Differences
Seifert et al. BMC Cancer (2015) 15:952 DOI 10.1186/s12885-015-1939-9 RESEARCH ARTICLE Open Access Comparative transcriptomics reveals similarities and differences between astrocytoma grades Michael Seifert1,2,5*, Martin Garbe1, Betty Friedrich1,3, Michel Mittelbronn4 and Barbara Klink5,6,7 Abstract Background: Astrocytomas are the most common primary brain tumors distinguished into four histological grades. Molecular analyses of individual astrocytoma grades have revealed detailed insights into genetic, transcriptomic and epigenetic alterations. This provides an excellent basis to identify similarities and differences between astrocytoma grades. Methods: We utilized public omics data of all four astrocytoma grades focusing on pilocytic astrocytomas (PA I), diffuse astrocytomas (AS II), anaplastic astrocytomas (AS III) and glioblastomas (GBM IV) to identify similarities and differences using well-established bioinformatics and systems biology approaches. We further validated the expression and localization of Ang2 involved in angiogenesis using immunohistochemistry. Results: Our analyses show similarities and differences between astrocytoma grades at the level of individual genes, signaling pathways and regulatory networks. We identified many differentially expressed genes that were either exclusively observed in a specific astrocytoma grade or commonly affected in specific subsets of astrocytoma grades in comparison to normal brain. Further, the number of differentially expressed genes generally increased with the astrocytoma grade with one major exception. The cytokine receptor pathway showed nearly the same number of differentially expressed genes in PA I and GBM IV and was further characterized by a significant overlap of commonly altered genes and an exclusive enrichment of overexpressed cancer genes in GBM IV. Additional analyses revealed a strong exclusive overexpression of CX3CL1 (fractalkine) and its receptor CX3CR1 in PA I possibly contributing to the absence of invasive growth. -
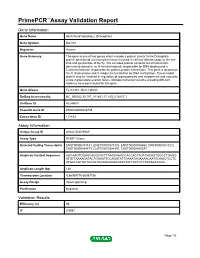
Download Validation Data
PrimePCR™Assay Validation Report Gene Information Gene Name dachshund homolog 2 (Drosophila) Gene Symbol DACH2 Organism Human Gene Summary This gene is one of two genes which encode a protein similar to the Drosophila protein dachshund a transcription factor involved in cell fate determination in the eye limb and genital disc of the fly. The encoded protein contains two characteristic dachshund domains: an N-terminal domain responsible for DNA binding and a C-terminal domain responsible for protein-protein interactions. This gene is located on the X chromosome and is subject to inactivation by DNA methylation. The encoded protein may be involved in regulation of organogenesis and myogenesis and may play a role in premature ovarian failure. Multiple transcript variants encoding different isoforms have been found for this gene. Gene Aliases FLJ31391, MGC138545 RefSeq Accession No. NC_000023.10, NT_011651.17, NG_012817.1 UniGene ID Hs.86603 Ensembl Gene ID ENSG00000126733 Entrez Gene ID 117154 Assay Information Unique Assay ID qHsaCID0009489 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000373131, ENST00000373125, ENST00000508860, ENST00000510272, ENST00000484479, ENST00000344497, ENST00000400297 Amplicon Context Sequence AGCAAGTGGAGCAGGCACTTAAGCAAGCCACCACTAGTGACAGTGGCCTGAGG ATGTTAAAAGATACTGGAATTCCAGATATTGAAATAGAAAACAATGGGACTCCTC ATGATAGTGCTGCTATGCAAGGAGGTAACTATTACTGTTTAGAAATGGC Amplicon Length (bp) 126 Chromosome Location X:86069775-86087136 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) -

Nº Ref Uniprot Proteína Péptidos Identificados Por MS/MS 1 P01024
Document downloaded from http://www.elsevier.es, day 26/09/2021. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Nº Ref Uniprot Proteína Péptidos identificados 1 P01024 CO3_HUMAN Complement C3 OS=Homo sapiens GN=C3 PE=1 SV=2 por 162MS/MS 2 P02751 FINC_HUMAN Fibronectin OS=Homo sapiens GN=FN1 PE=1 SV=4 131 3 P01023 A2MG_HUMAN Alpha-2-macroglobulin OS=Homo sapiens GN=A2M PE=1 SV=3 128 4 P0C0L4 CO4A_HUMAN Complement C4-A OS=Homo sapiens GN=C4A PE=1 SV=1 95 5 P04275 VWF_HUMAN von Willebrand factor OS=Homo sapiens GN=VWF PE=1 SV=4 81 6 P02675 FIBB_HUMAN Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 78 7 P01031 CO5_HUMAN Complement C5 OS=Homo sapiens GN=C5 PE=1 SV=4 66 8 P02768 ALBU_HUMAN Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 66 9 P00450 CERU_HUMAN Ceruloplasmin OS=Homo sapiens GN=CP PE=1 SV=1 64 10 P02671 FIBA_HUMAN Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 58 11 P08603 CFAH_HUMAN Complement factor H OS=Homo sapiens GN=CFH PE=1 SV=4 56 12 P02787 TRFE_HUMAN Serotransferrin OS=Homo sapiens GN=TF PE=1 SV=3 54 13 P00747 PLMN_HUMAN Plasminogen OS=Homo sapiens GN=PLG PE=1 SV=2 48 14 P02679 FIBG_HUMAN Fibrinogen gamma chain OS=Homo sapiens GN=FGG PE=1 SV=3 47 15 P01871 IGHM_HUMAN Ig mu chain C region OS=Homo sapiens GN=IGHM PE=1 SV=3 41 16 P04003 C4BPA_HUMAN C4b-binding protein alpha chain OS=Homo sapiens GN=C4BPA PE=1 SV=2 37 17 Q9Y6R7 FCGBP_HUMAN IgGFc-binding protein OS=Homo sapiens GN=FCGBP PE=1 SV=3 30 18 O43866 CD5L_HUMAN CD5 antigen-like OS=Homo -

Epigenetic Analysis of the Critical Region I for Premature Ovarian Failure
Original article Epigenetic analysis of the critical region I for J Med Genet: first published as 10.1136/jmg.2007.056093 on 15 July 2008. Downloaded from premature ovarian failure: demonstration of a highly heterochromatic domain on the long arm of the mammalian X chromosome F Rizzolio,1 T Pramparo,3 C Sala,1 O Zuffardi,3 L De Santis,5 E Rabellotti,5 F Calzi,5 F Fusi,5 R Bellazzi,4 D Toniolo1,2 c Additional tables and figures ABSTRACT deletions of CR1 were not. These findings suggested are published online only at Background: X chromosome rearrangements defined a that POF CR1 may not carry genes for ovarian http://jmg.bmj.com/content/ critical region for premature ovarian failure (POF) that vol46/issue9 function as previously hypothesised and that such extended for .15 Mb in Xq. It has been shown genes should be present only in CR2. Indeed, 1 DIBIT, San Raffaele Scientific previously that the region could be divided into two mapping of several breakpoints in the region failed Institute, Milano, Italy; 2 Institute of Molecular Genetics, CNR, functionally distinct portions and suggested that balanced to identify ovary expressed genes that could be Pavia, Italy; 3 Department of translocations interrupting its proximal part, critical region responsible for POF. Such genes were found at the Pathology and Medical Genetics, 1 (CR1), could be responsible for POF through down- autosomal breakpoints, in six cases studied.9 University of Pavia, Pavia, Italy; 4 regulation of ovary expressed autosomal genes translo- Based on our data we suggested that POF CR1 Department of Computer cated to the X chromosome. -

A Novel Chromosomal Translocation Identified Due to Complex Genetic
cells Article A Novel Chromosomal Translocation Identified due to Complex Genetic Instability in iPSC Generated for Choroideremia 1,2 3, 1,2, 3 Nejla Erkilic , Vincent Gatinois y , Simona Torriano y, Pauline Bouret , Carla Sanjurjo-Soriano 1,2, Valerie De Luca 1,2, Krishna Damodar 1,2, Nicolas Cereso 1,2, Jacques Puechberty 4, Rocio Sanchez-Alcudia 5,6, Christian P. Hamel 1,2,7, Carmen Ayuso 5,6, 1,2,7 3, 1,2, , Isabelle Meunier , Franck Pellestor z and Vasiliki Kalatzis * z 1 Inserm U1051, Institute for Neurosciences of Montpellier, 34091 Montpellier, France 2 University of Montpellier, 34095 Montpellier, France 3 Chromosomal Genetics Unit, Chromostem Platform, CHU, 34090 Montpellier, France 4 Service of Clinical Genetics, Department of Medical Genetics, Rare Diseases and Personalized Medicine, CHU, 34090 Montpellier, France 5 Department of Genetics, Institute for Sanitary Investigation, Foundation Jimenez Diaz, 28040 Madrid, Spain 6 Centre for Biomedical Network Research on Rare Diseases (CIBERER), 28029 Madrid, Spain 7 National Reference Centre for Inherited Sensory Diseases, CHU, 34295 Montpellier, France * Correspondence: [email protected]; Tel.: +33-(0)4-99-63-6076; Fax: +33-(0)4-99-63-6020 These authors contributed equally to this work. y These authors contributed equally to this work. z Received: 13 August 2019; Accepted: 7 September 2019; Published: 11 September 2019 Abstract: Induced pluripotent stem cells (iPSCs) have revolutionized the study of human diseases as they can renew indefinitely, undergo multi-lineage differentiation, and generate disease-specific models. However, the difficulty of working with iPSCs is that they are prone to genetic instability. Furthermore, genetically unstable iPSCs are often discarded, as they can have unforeseen consequences on pathophysiological or therapeutic read-outs. -

Genetics of Premature Ovarian Insufficiency and the Association with X-Autosome Translocations
160 4 REPRODUCTIONREVIEW Genetics of premature ovarian insufficiency and the association with X-autosome translocations Adriana Di-Battista, Mariana Moysés-Oliveira and Maria Isabel Melaragno Genetics Division, Department of Morphology and Genetics, Universidade Federal de São Paulo, São Paulo, Brazil Correspondence should be addressed to M I Melaragno; Email: [email protected] Abstract Premature ovarian insufficiency (POI) is the cessation of menstruation before the age of 40 and can result from different etiologies, including genetic, autoimmune, and iatrogenic. Of the genetic causes, single-gene mutations and cytogenetic alterations, such as X-chromosome aneuploidies and chromosome rearrangements, can be associated with POI. In this review, we summarize the genetic factors linked to POI and list the main candidate genes. We discuss the association of these genes with the ovarian development, the functional consequences of different mutational mechanisms and biological processes that are frequently disrupted during POI pathogenesis. Additionally, we focus on the high prevalence of X-autosome translocations involving the critical regions in Xq, known as POI1 and POI2, and discuss in depth the main hypotheses proposed to explain this association. Although the incorrect pairing of chromosomes during meiosis could lead to oocyte apoptosis, the reason for the prevalence of X-chromosome breakpoints at specific regions remains unclear. In most cases, studies on genes disrupted by balanced structural rearrangements cannot explain the ovarian failure. Thus, the position effect has emerged as a putative explanation for genetic mechanisms as translocations possibly result in changes in overall chromatin topology due to chromosome repositioning. Given the tremendous impact of POI on women’s quality of life, we highlight the value of investigations in to the interplay between ovarian function and gene regulation to deepen our understanding of the molecular mechanisms related to this disease, with the ultimate goal of improving patients’ care and assistance. -

Whole-Exome Analysis Reveals X-Linked Recessive Mutations Underlying Premature Ovarian Failure Using Galaxy Platform Abstract A
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 21 May 2021 doi:10.20944/preprints202105.0519.v1 Whole-Exome Analysis Reveals X-linked Recessive Mutations underlying Premature Ovarian Failure using Galaxy Platform Rashid Saif1*, Tania Mahmood1, Aniqa Ejaz1, Saeeda Zia2, Saqer Sultan Alotaibi3 1Decode Genomics, 323-D, Punjab University Employees Housing Scheme (II), Lahore, Pakistan 2Department of Sciences and Humanities, National University of Computer and Emerging Sciences, Lahore, Pakistan 3Department of Biotechnology, College of Science, Taif University, Taif 21944, Saudi Arabia *Corresponding Author: [email protected] Abstract An in-silico WES approach using the Galaxy platform was adopted in the current study to predict the genetic basis of Premature Ovarian Failure (POF), where three affected patients in a Saudi Arabian family of seven, found associated with X-linked recessive mutations. The current analysis discovered 518,054 variants using FreeBayes variant caller that had 1,461,864 effects on variable sites in the genome revealed by SnpEff software. The causal genetic mutations were filtered and annotated with the ClinVar database using the GEMINI tool. This tool retained 369 pathogenic mutations harboring 130 genes. Among the total, 268 variants positioned on 69 genes are shared with three affected individuals, 61 variants on 23 genes are shared by any two of the affected individuals, and 40 of the variants on 38 genes are present in any one of the affected sample. Two mutations in one of the already POF-associated, POF1B gene were also observed e.g. (i) g.84563135T>A; p.M349L and (ii) g.84563194C>T; p.R329Q in the two affected individuals i.e. -

High-Density Array Comparative Genomic Hybridization Detects Novel Copy Number Alterations in Gastric Adenocarcinoma
ANTICANCER RESEARCH 34: 6405-6416 (2014) High-density Array Comparative Genomic Hybridization Detects Novel Copy Number Alterations in Gastric Adenocarcinoma ALINE DAMASCENO SEABRA1,2*, TAÍSSA MAÍRA THOMAZ ARAÚJO1,2*, FERNANDO AUGUSTO RODRIGUES MELLO JUNIOR1,2, DIEGO DI FELIPE ÁVILA ALCÂNTARA1,2, AMANDA PAIVA DE BARROS1,2, PAULO PIMENTEL DE ASSUMPÇÃO2, RAQUEL CARVALHO MONTENEGRO1,2, ADRIANA COSTA GUIMARÃES1,2, SAMIA DEMACHKI2, ROMMEL MARIO RODRÍGUEZ BURBANO1,2 and ANDRÉ SALIM KHAYAT1,2 1Human Cytogenetics Laboratory and 2Oncology Research Center, Federal University of Pará, Belém Pará, Brazil Abstract. Aim: To investigate frequent quantitative alterations gastric cancer is the second most frequent cancer in men and of intestinal-type gastric adenocarcinoma. Materials and the third in women (4). The state of Pará has a high Methods: We analyzed genome-wide DNA copy numbers of 22 incidence of gastric adenocarcinoma and this disease is a samples and using CytoScan® HD Array. Results: We identified public health problem, since mortality rates are above the 22 gene alterations that to the best of our knowledge have not Brazilian average (5). been described for gastric cancer, including of v-erb-b2 avian This tumor can be classified into two histological types, erythroblastic leukemia viral oncogene homolog 4 (ERBB4), intestinal and diffuse, according to Laurén (4, 6, 7). The SRY (sex determining region Y)-box 6 (SOX6), regulator of intestinal type predominates in high-risk areas, such as telomere elongation helicase 1 (RTEL1) and UDP- Brazil, and arises from precursor lesions, whereas the diffuse Gal:betaGlcNAc beta 1,4- galactosyltransferase, polypeptide 5 type has a similar distribution in high- and low-risk areas and (B4GALT5). -

Ancestral Regulatory Mechanisms Specify Conserved Midbrain Circuitry in Arthropods and Vertebrates
Ancestral regulatory mechanisms specify conserved midbrain circuitry in arthropods and vertebrates Jessika C. Bridia,b,1,2, Zoe N. Ludlowa,b,1, Benjamin Kottlera,b, Beate Hartmannc, Lies Vanden Broeckd, Jonah Dearlovea,b, Markus Gökere, Nicholas J. Strausfeldf,g,3, Patrick Callaertsd, and Frank Hirtha,b,3 aDepartment of Basic and Clinical Neuroscience, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 9RT, United Kingdom; bDepartment of Basic and Clinical Neuroscience, Maurice Wohl Clinical Neuroscience Institute, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London SE5 9RT, United Kingdom; cUniversity of Basel, 4031 Basel, Switzerland; dLaboratory of Behavioural & Developmental Genetics, Department of Human Genetics, Katholieke Universiteit Leuven, 6023000 Leuven, Belgium; eLeibniz Institute German Collection of Microorganisms and Cell Cultures, 38124 Braunschweig, Germany; fDepartment of Neuroscience, University of Arizona, Tucson, AZ 85721; and gCenter for Insect Sciences, University of Arizona, Tucson, AZ 85721 Edited by Michael Levine, Princeton University, Princeton, NJ, and approved June 9, 2020 (received for review October 26, 2019) Corresponding attributes of neural development and function (MHB) development (20). Further cross-phyletic studies revealed suggest arthropod and vertebrate brains may have an evolution- correspondences in developmental genetic mechanisms underly- arily conserved organization. However, the underlying mecha- ing circuit formation and -

DACH2 Rabbit Polyclonal Antibody – TA349865 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TA349865 DACH2 Rabbit Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: IHC Recommended Dilution: ELISA: 2000-5000, IHC: 50-200 Reactivity: Human, Mouse Host: Rabbit Isotype: IgG Clonality: Polyclonal Immunogen: Fusion protein of human DACH2 Formulation: pH7.4 PBS, 0.05% NaN3, 40% Glyceroln Concentration: lot specific Purification: Antigen affinity purification Conjugation: Unconjugated Storage: Store at -20°C as received. Stability: Stable for 12 months from date of receipt. Gene Name: dachshund family transcription factor 2 Database Link: NP_444511 Entrez Gene 93837 MouseEntrez Gene 117154 Human Q96NX9 Background: This gene is one of two genes which encode a protein similar to the Drosophila protein dachshund, a transcription factor involved in cell fate determination in the eye, limb and genital disc of the fly. The encoded protein contains two characteristic dachshund domains: an N-terminal domain responsible for DNA binding and a C-terminal domain responsible for protein-protein interactions. This gene is located on the X chromosome and is subject to inactivation by DNA methylation. The encoded protein may be involved in regulation of organogenesis and myogenesis, and may play a role in premature ovarian failure. Multiple transcript variants encoding different isoforms have been found for this gene. Synonyms: FLJ31391; MGC138545 Protein Families: Transcription Factors This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 2 DACH2 Rabbit Polyclonal Antibody – TA349865 Product images: The image on the left is immunohistochemistry of paraffin-embedded Human thyroid cancer tissue using (DACH2 Antibody) at dilution 1/40, on the right is treated with fusion protein. -

Breakpoint Determination of X;Autosome Balanced Translocations in Four Patients with Premature Ovarian Failure
Journal of Human Genetics (2011) 56, 156–160 & 2011 The Japan Society of Human Genetics All rights reserved 1434-5161/11 $32.00 www.nature.com/jhg ORIGINAL ARTICLE Breakpoint determination of X;autosome balanced translocations in four patients with premature ovarian failure Akira Nishimura-Tadaki1, Takahito Wada2, Gul Bano3, Karen Gough4, Janet Warner4, Tomoki Kosho5, Noriko Ando6, Haruka Hamanoue1,7, Hideya Sakakibara7, Gen Nishimura8, Yoshinori Tsurusaki1, Hiroshi Doi1, Noriko Miyake1, Keiko Wakui5, Hirotomo Saitsu1, Yoshimitsu Fukushima5, Fumiki Hirahara7 and Naomichi Matsumoto1 Premature ovarian failure (POF) is a disorder characterized by amenorrhea and elevated serum gonadotropins before 40 years of age. As X chromosomal abnormalities are often recognized in POF patients, defects of X-linked gene may contribute to POF. Four cases of POF with t(X;autosome) were genetically analyzed. All the translocation breakpoints were determined at the nucleotide level. Interestingly, COL4A6 at Xq22.3 encoding collagen type IV alpha 6 was disrupted by the translocation in one case, but in the remaining three cases, breakpoints did not involve any X-linked genes. According to the breakpoint sequences, two translocations had microhomology of a few nucleotides and the other two showed insertion of 3–8 nucleotides with unknown origin, suggesting that non-homologous end-joining is related to the formation of all the translocations. Journal of Human Genetics (2011) 56, 156–160; doi:10.1038/jhg.2010.155; published online 9 December 2010 Keywords: COL4A6; critical region; non-homologous end-joining; premature ovarian failure; X;autosome translocation INTRODUCTION translocations. Breakpoint sequences will be presented and discussed Premature ovarian failure (POF) is a disorder characterized by in relation to genes and formation process.