Callicorixa Vulnerata)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Position Specificity in the Genus Coreomyces (Laboulbeniomycetes, Ascomycota)
VOLUME 1 JUNE 2018 Fungal Systematics and Evolution PAGES 217–228 doi.org/10.3114/fuse.2018.01.09 Position specificity in the genus Coreomyces (Laboulbeniomycetes, Ascomycota) H. Sundberg1*, Å. Kruys2, J. Bergsten3, S. Ekman2 1Systematic Biology, Department of Organismal Biology, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden 2Museum of Evolution, Uppsala University, Uppsala, Sweden 3Department of Zoology, Swedish Museum of Natural History, Stockholm, Sweden *Corresponding author: [email protected] Key words: Abstract: To study position specificity in the insect-parasitic fungal genus Coreomyces (Laboulbeniaceae, Laboulbeniales), Corixidae we sampled corixid hosts (Corixidae, Heteroptera) in southern Scandinavia. We detected Coreomyces thalli in five different DNA positions on the hosts. Thalli from the various positions grouped in four distinct clusters in the resulting gene trees, distinctly Fungi so in the ITS and LSU of the nuclear ribosomal DNA, less so in the SSU of the nuclear ribosomal DNA and the mitochondrial host-specificity ribosomal DNA. Thalli from the left side of abdomen grouped in a single cluster, and so did thalli from the ventral right side. insect Thalli in the mid-ventral position turned out to be a mix of three clades, while thalli growing dorsally grouped with thalli from phylogeny the left and right abdominal clades. The mid-ventral and dorsal positions were found in male hosts only. The position on the left hemelytron was shared by members from two sister clades. Statistical analyses demonstrate a significant positive correlation between clade and position on the host, but also a weak correlation between host sex and clade membership. These results indicate that sex-of-host specificity may be a non-existent extreme in a continuum, where instead weak preferences for one host sex may turn out to be frequent. -

UBC 1978 A6 7 C35.Pdf
THE INFLUENCE OF TEMPERATURE AND SALINITY ON THE CUTICULAR PERMEABILITY OF SOME CORIXIDAE by SYDNEY GRAHAM CANNINGS B.Sc, University of British Columbia, 1975 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES (Department of Zoology) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA November, 19 77 © Sydney Graham Cannings, 1977 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of ZOOLOGY The University of British Columbia 2075 Wesbrook Place Vancouver, Canada V6T 1W5 Date November 14, 1977 ABSTRACT Most terrestrial, and many aquatic insects are made waterproof by a layer of lipid in or on the epicuticle. At a specific temperature, which is determined by their composition, these lipids undergo a phase .transition which markedly increases the permeability of the integument. The major purpose of this study was to assess the possibility that epicutic.ular wax transition could differ• entially affect the distribution of four species of water boatmen: Cenocorixa bifida hungerfordi Lansbury, Ceno- corixa expleta (Uhler), Cenocorixa blaisdelli. (Hunger- ford) , and Callicorixa vulnerata (Uhler). -
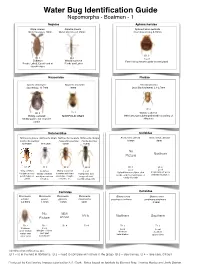
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

CBD Fifth National Report
CONVENTION ON CONVENTION ON BIOLOGICAL DIVERSITY BIOLOGICAL DIVERSITY THE 5TH NATIONAL REPORT OF MONGOLIA biolohJA JJa folea YeehcO beiide& oa KnWWn}A. T HE CONVENTION ON BIOLOGI 5 T H N A T IO N AL R EPO RT C AL DIVERSITY OF M O N GOLIA MINISTRY OF ENVIRONMENT AND GREEN DEVELOPMENT STEPPE FORWARD PROGRAMME, Government building II, BIOLOGY DEPARTMENT, United Nation’s street 5/2, NATIONAL UNIVERSITY OF MONGOLIA TH Chingeltei District, Ulaanbaatar 15160, NUM, Building-2, Ulaanbaatar, Mongolia THE 5 NATIONAL REPORT OF Mongolia P.O.Box 537, Ulaanbaatar 210646A, Tel: 976-51-266197 Ulaanbaatar, Mongolia E-mail: [email protected] Tel: 976-99180148; 976-88305909; 976-88083058 MONGOLIA E-mail: [email protected]; [email protected]; [email protected] Designed by Mongolica Publishing 2014 Ulaanbaatar, Mongolia. 2014 CONVENTION ON BIOLOGICAL DIVERSITY CONVENTION ON BIOLOGICAL DIVERSITY FINANCED BY: MINISTRY OF ENVIRONMENT AND GREEN DEVELOPMENT CONVENTION ON BIOLOGICAL DIVERSITY-MONGOLIA GLOBAL ENVIRONMENT FACILITY UNITED NATIONS ENVIRONMENTAL PROGRAM CONVENTION ON BIOLOGICAL DIVERSITY THE 5TH NATIONAL REPORT OF MONGOLIA REPORT COMPILERS: COMPILED BY: S. GOMBOBAATAR STEPPE FORWARD PROGRAMME, NUM S. MYAGMARSUREN N. CONABOY М. Мunkhjargal TAXON COMPILERS: PLANT: B. OYUNTSETSEG, M. URGAMAL INVERTEBRATE: S. GANTIGMAA Fish, aMphibian, reptile: kh. Тerbish BIRD: S. GOMBOBAATAR MAMMAL: S. SHAR CONTRIBUTIONS FROM: EDITORS: NATIONAL UNIVERSITY OF MONGOLIA INSTITUTE OF BIOLOGY, MONGOLIAN ACADEMY OF SCIENCES D. BATBOLD MONGOLIAN ORNITHOLOGICAL SOCIETY -

Beiträge Zur Bayerischen Entomofaunistik 13: 67–207
Beiträge zur bayerischen Entomofaunistik 13:67–207, Bamberg (2014), ISSN 1430-015X Grundlegende Untersuchungen zur vielfältigen Insektenfauna im Tiergarten Nürnberg unter besonderer Betonung der Hymenoptera Auswertung von Malaisefallenfängen in den Jahren 1989 und 1990 von Klaus von der Dunk & Manfred Kraus Inhaltsverzeichnis 1. Einleitung 68 2. Untersuchungsgebiet 68 3. Methodik 69 3.1. Planung 69 3.2. Malaisefallen (MF) im Tiergarten 1989, mit Gelbschalen (GS) und Handfänge 69 3.3. Beschreibung der Fallenstandorte 70 3.4. Malaisefallen, Gelbschalen und Handfänge 1990 71 4. Darstellung der Untersuchungsergebnisse 71 4.1. Die Tabellen 71 4.2. Umfang der Untersuchungen 73 4.3. Grenzen der Interpretation von Fallenfängen 73 5. Untersuchungsergebnisse 74 5.1. Hymenoptera 74 5.1.1. Hymenoptera – Symphyta (Blattwespen) 74 5.1.1.1. Tabelle Symphyta 74 5.1.1.2. Tabellen Leerungstermine der Malaisefallen und Gelbschalen und Blattwespenanzahl 78 5.1.1.3. Symphyta 79 5.1.2. Hymenoptera – Terebrantia 87 5.1.2.1. Tabelle Terebrantia 87 5.1.2.2. Tabelle Ichneumonidae (det. R. Bauer) mit Ergänzungen 91 5.1.2.3. Terebrantia: Evanoidea bis Chalcididae – Ichneumonidae – Braconidae 100 5.1.2.4. Bauer, R.: Ichneumoniden aus den Fängen in Malaisefallen von Dr. M. Kraus im Tiergarten Nürnberg in den Jahren 1989 und 1990 111 5.1.3. Hymenoptera – Apocrita – Aculeata 117 5.1.3.1. Tabellen: Apidae, Formicidae, Chrysididae, Pompilidae, Vespidae, Sphecidae, Mutillidae, Sapygidae, Tiphiidae 117 5.1.3.2. Apidae, Formicidae, Chrysididae, Pompilidae, Vespidae, Sphecidae, Mutillidae, Sapygidae, Tiphiidae 122 5.1.4. Coleoptera 131 5.1.4.1. Tabelle Coleoptera 131 5.1.4.2. -

(Tropocorixa) Jensenhaarupi (Hemiptera: Heteroptera: Corixidae: Corixini), with Ecological Notes Revista Mexicana De Biodiversidad, Vol
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Melo, María Cecilia; Scheibler, Erica Elizabeth Description of the immature stages ofSigara (Tropocorixa) jensenhaarupi (Hemiptera: Heteroptera: Corixidae: Corixini), with ecological notes Revista Mexicana de Biodiversidad, vol. 82, núm. 1, marzo, 2011, pp. 117-130 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42520745011 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Biodiversidad 82: 117-130, 2011 Description of the immature stages of Sigara (Tropocorixa) jensenhaarupi (Hemiptera: Heteroptera: Corixidae: Corixini), with ecological notes Descripción de los estadios larvales de Sigara (Tropocorixa) jensenhaarupi (Heteroptera: Corixidae), con notas acerca de su ecología María Cecilia Melo1* and Erica Elizabeth Scheibler2 1Departamento Sistemática, Instituto de Limnología “R.A. Ringuelet” (ILPLA) (CCT La Plata CONICET- UNLP), C.C. 712, 1900 La Plata, Argentina. 2Laboratorio de Entomología, Instituto Argentino de Investigaciones de las Zonas Áridas (IADIZA, CCT CONICET, Mendoza), C.C. 507, 5500 Men- doza, Argentina. *Correspondent: [email protected] Abstract. Sigara (Tropocorixa) jensenhaarupi Jaczewski is the smallest species of the subgenus ranging from 4.2-4.7 mm, and it is characterized by the absence of a strigil, the small and narrow genital capsule with a short hypandrium in males, and the shape of the abdominal tergite VII in females. This species is endemic to the Patagonian subregion (Andean region) in Argentina. -

Cytogenetic Characterization of Three Species of Sigara from Argentina and the Plausible Mechanisms of Karyotype Evolution Within Nepomorpha
ISSN 0373-5680 Rev. Soc. Entomol. Argent. 66 (3-4): 81-89, 2007 81 New contributions to the study of Corixoidea: cytogenetic characterization of three species of Sigara from Argentina and the plausible mechanisms of karyotype evolution within Nepomorpha BRESSA, María José and Alba Graciela PAPESCHI Laboratorio de Citogenética y Evolución, Departamento de Ecología, Genética y Evolución, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria. Pabellón 2, C1428EGA, Ciudad Autónoma de Buenos Aires, Argentina; e-mail: [email protected] Nuevas contribuciones al estudio de Corixoidea: caracterización citogenética de tres especies de Sigara de Argentina y los posibles mecanismos de evolución del cariotipo en Nepomorpha RESUMEN. Los estudios citogenéticos en Heteroptera contribuyen al análisis de las tendencias evolutivas en el taxón. Los Heteroptera se caracterizan por poseer cromosomas holocinéticos, diferentes sistemas de cromosomas sexuales y un par de cromosomas m en algunas especies. En este trabajo describimos el cariotipo y la meiosis masculina de Sigara denseconscripta (Breddin), S. chrostowskii Jaczewski y S. rubyae (Hungerford). Las tres especies tienen un número diploide de 24, con un par de cromosomas m y un sistema de cromosomas sexuales XY/XX. Con estos resultados son 30 las especies de Corixoidea estudiadas citogenéticamente y el cariotipo modal de la superfamilia es 2n= 20+2m+XY en machos. La información citogenética disponible hasta el presente en Heteroptera nos permite sugerir que la presencia de cromosomas m y cromosomas sexuales XY/XX, serían caracteres plesiomórficos para Nepomorpha. La ausencia de cromosomas m en especies de Nepoidea y Ochteroidea, y los sistemas de cromosomas sexuales X0 y Xn0 (en machos) en especies de Corixoidea, Naucoroidea y Nepoidea, serían caracteres derivados que habrían surgido evolutivamente más tarde. -

Hemiptera: Heteroptera) from India
Check-list of Fauna of India CHECK LIST OF NEPOMORPHA (HEMIPTERA: HETEROPTERA) FROM INDIA G.Thirumalai Southern Regional Station, Zoological Survey of India, Chennai - 600 028 Email: [email protected] ORDER : HEMIPTERA SUBORDER : HETEROPTERA INFRAORDER : NEPOMORPHA POPOV, 1968 Superfamily: Nepoidea Latreille, 1802 FAMILY : NEPIDAE LATREILLE, 1802 Subfamily : Nepinae Latreille, 1802 Tribe : Nepini Latreille, 1802 Genus : Laccotrephes Stål, 1866 Subgenus : Laccotrephes Stål, 1866 1. Laccotrephes elongatus Montandon , 1907 2. Laccotrephes fabricii fabricii Stål , 1868 3. Laccotrephes griseus (Gúerin-Méneville , 1844) 4. Laccotrephes grossus (Fabricius, 1787) 5. Laccotrephes maculatus (Fabricius, 1775) 6. Laccotrephes pfeiferiae (Ferrari, 1888) 7. Laccotrephes ruber (Linnaeus, 1764) Genus : Montonepa Lansbury, 1973 8. Montonepa erutus (Montandon, 1910) Subfamily : Ranatrinae Douglas & Scott, 1865 Tribe : Ranatrini Douglas & Scott, 1865 Genus : Cercotmetus Amyot & Serville, 1843 9. Cercotmetus asiaticus Amyot & Serville, 1843 10. Cercotmetus brevipes Montandon, 1909 11. Cercotmetus fumosus Distant, 1904 12. Cercotmetus pilipes (Dallas) 1850 Genus : Ranatra Fabricius, 1790 Subgenus : Ranatra Fabricius, 1790 13. Ranatra chinensis Mayr, 1865 14. Ranatra digitata Hafiz & Pradhan , 1947 15. Ranatra distanti Montandon , 1910 16. Ranatra elongata Fabricius , 1790 17. Ranatra feana Montandon, 1903 18. Ranatra filiformis Fabricius, 1790 19. Ranatra gracilis Dallas , 1850 20. Ranatra longipes thai Lansbury , 1972 21. Ranatra parmata Mayr ,1865 22. -

Het News Issue 3
Issue 3 Spring 2004 Het News nd 2 Series Newsletter of the Heteroptera Recording Schemes Editorial: There is a Dutch flavour to this issue which we hope will be of interest. After all, The Netherlands is not very far as the bug flies and with a following wind there could easily be immigrants reaching our shores at any time. We have also introduced an Archive section, for historical articles, to appear when space allows. As always we are very grateful to all the providers of material for this issue and, for the next issue, look forward to hearing about your 2004 (& 2003) exploits, exciting finds, regional news, innovative gadgets etc. Sheila Brooke 18 Park Hill Toddington Dunstable Beds LU5 6AW [email protected] Bernard Nau 15 Park Hill Toddington Dunstable Beds LU5 6AW [email protected] Contents Editorial .................................................................... 1 Forthcoming & recent events ................................. 7 Dutch Bug Atlas....................................................... 1 Checklist of British water bugs .............................. 8 Recent changes in the Dutch Heteroptera............. 2 The Lygus situation............................................... 11 Uncommon Heteroptera from S. England ............. 5 Web Focus.............................................................. 12 News from the Regions ........................................... 6 From the Archives ................................................. 12 Gadget corner – Bug Mailer..................................... -

Community Structure and Secondary Production of Aquatic Macroinvertebrates in Coastal Wetland Ponds of the West Copper River Delta, Alaska, Following Tectonic Uplift
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 2011 Community Structure and Secondary Production of Aquatic Macroinvertebrates in Coastal Wetland Ponds of the West Copper River Delta, Alaska, Following Tectonic Uplift Ryan Glen Van Duzor Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Terrestrial and Aquatic Ecology Commons Recommended Citation Van Duzor, Ryan Glen, "Community Structure and Secondary Production of Aquatic Macroinvertebrates in Coastal Wetland Ponds of the West Copper River Delta, Alaska, Following Tectonic Uplift" (2011). Master's Theses. 560. https://ecommons.luc.edu/luc_theses/560 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 2011 Ryan Glen Van Duzor LOYOLA UNIVERSITY CHICAGO COMMUNITY STRUCTURE AND SECONDARY PRODUCTION OF AQUATIC MACROINVERTEBRATES IN COASTAL WETLAND PONDS OF THE WEST COPPER RIVER DELTA, ALASKA, FOLLOWING TECTONIC UPLIFT A THESIS SUBMITTED TO THE FACULTY OF THE GRADUATE SCHOOL IN CANDIDACY FOR THE DEGREE OF MASTER OF SCIENCE PROGRAM IN BIOLOGY BY RYAN G. VAN DUZOR CHICAGO, IL MAY 2011 Copyright© 2011 by Ryan Glen Van Duzor All rights reserved ACKNOWLEDGMENTS First of all, I would like to thank Dr. Martin Berg for his steadfast advising, mentorship and all around friendship. I cannot begin to express how honored I am to have worked closely with him and how fortunate I feel that I was able to do my research in a place as amazing as the Copper River Delta, Alaska. -

The Diversity of Feeding Habits Recorded for Water Boatmen
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 114: 147–159, 2017 http://www.eje.cz doi: 10.14411/eje.2017.020 REVIEW The diversity of feeding habits recorded for water boatmen (Heteroptera: Corixoidea) world-wide with implications for evaluating information on the diet of aquatic insects CHRISTIAN W. HÄDICKE 1, *, DÁVID RÉDEI 2, 3 and PETR KMENT 4 1 University of Rostock, Institute of Biosciences, Chair of Zoology, Universitätsplatz 2, 18055 Rostock, Germany 2 Institute of Entomology, College of Life Sciences, Nankai University, Weijin Rd. 94, 300071 Tianjin, China; e-mail: [email protected] 3 Hungarian Natural History Museum, Department of Zoology, 1088 Budapest, Baross u. 13, Hungary 4 National Museum, Department of Entomology, Cirkusová 1740, CZ-193 00 Praha 9 – Horní Počernice, Czech Republic; e-mail: [email protected] Key words. Heteroptera, Corixoidea, aquatic invertebrates, food webs, diet, predation, feeding habits Abstract. Food webs are of crucial importance for understanding any ecosystem. The accuracy of food web and ecosystem models rests on the reliability of the information on the feeding habits of the species involved. Water boatmen (Corixoidea) is the most diverse superfamily of water bugs (Heteroptera: Nepomorpha), frequently the most abundant group of insects in a variety of freshwater habitats worldwide. In spite of their high biomass, the importance of water boatmen in aquatic ecosystems is fre- quently underestimated. The diet and feeding habits of Corixoidea are unclear as published data are frequently contradictory. We summarise information on the feeding habits of this taxon, which exemplify the diffi culties in evaluating published data on feeding habits in an invertebrate taxon.