UBC 1978 A6 7 C35.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE Fmmature STAGES of Cpf1o('Orixa Bilida (HUNG .) C. ('Xp
JOVHXAL El'iTO.\IOL. SOl'. BIlIT. COIXMI!IA, VOl.. 63 (1966), DEC. 1, 1966 THE fMMATURE STAGES OF Cpf1o('orixa bilida (HUNG .) AND C. ('xp!('/a (U HLEH) (HEMIPTERA: CORIXfDAE) G . G. E . SCUDDER Department of Zoology, University of British Columbia, Vancouver INTRODUCTION rately in the corresponding lake wat In a study of the ecology and er. In this way, C. bifida was reared physiology of Corixidae living in a to the adult instar ana C. expleta through the first three larval instars. series of soda lakes in central British Columbia, it was essential to identify All drawings have been made with a squared reticule eye-piece or Cam the larval instal'S of two species of era Lucida, us ing both compound and Cenocorixa, which often occur sym stereo-zoom microscopes. The spines patrically. This was necessary in or on the hind femora of larvae were der to work out the details of the life usually only clearly visible when legs cycle and be able to iden tify with were mounted in polyvinyl lactophe nol or other similar mountant and certainty, insects used in experi viewed at magnifications over 150x. ments. This paper describes the im mature stages of these l,WO species, The terminology and characters C. bifida (Hungerford) and C. expleta utilized in the larval descriptions fol (Uhler) . Both species have been pre low Cobben & Pillot (1960). However, viously recorded from British Colum I have interpreted the surfaces of the bia by Lansbury (1960) . legs differently. The surface of the hind leg seen in dorsal view is mor MATERIALS and METHODS phologically the posterior surface and The immature s tages were obtain is so interpreted. -

Position Specificity in the Genus Coreomyces (Laboulbeniomycetes, Ascomycota)
VOLUME 1 JUNE 2018 Fungal Systematics and Evolution PAGES 217–228 doi.org/10.3114/fuse.2018.01.09 Position specificity in the genus Coreomyces (Laboulbeniomycetes, Ascomycota) H. Sundberg1*, Å. Kruys2, J. Bergsten3, S. Ekman2 1Systematic Biology, Department of Organismal Biology, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden 2Museum of Evolution, Uppsala University, Uppsala, Sweden 3Department of Zoology, Swedish Museum of Natural History, Stockholm, Sweden *Corresponding author: [email protected] Key words: Abstract: To study position specificity in the insect-parasitic fungal genus Coreomyces (Laboulbeniaceae, Laboulbeniales), Corixidae we sampled corixid hosts (Corixidae, Heteroptera) in southern Scandinavia. We detected Coreomyces thalli in five different DNA positions on the hosts. Thalli from the various positions grouped in four distinct clusters in the resulting gene trees, distinctly Fungi so in the ITS and LSU of the nuclear ribosomal DNA, less so in the SSU of the nuclear ribosomal DNA and the mitochondrial host-specificity ribosomal DNA. Thalli from the left side of abdomen grouped in a single cluster, and so did thalli from the ventral right side. insect Thalli in the mid-ventral position turned out to be a mix of three clades, while thalli growing dorsally grouped with thalli from phylogeny the left and right abdominal clades. The mid-ventral and dorsal positions were found in male hosts only. The position on the left hemelytron was shared by members from two sister clades. Statistical analyses demonstrate a significant positive correlation between clade and position on the host, but also a weak correlation between host sex and clade membership. These results indicate that sex-of-host specificity may be a non-existent extreme in a continuum, where instead weak preferences for one host sex may turn out to be frequent. -
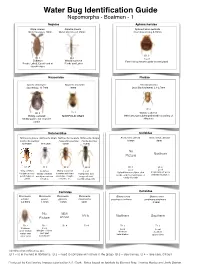
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

The Role of Freshwater Bioacoustics in Ecological Research
Greenhalgh, J., Genner, M. J., Jones, G., & Desjonquères, C. (2020). The role of freshwater bioacoustics in ecological research. Wiley Interdisciplinary Reviews: Water, [e1416]. https://doi.org/10.1002/wat2.1416 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1002/wat2.1416 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Wiley at https://onlinelibrary.wiley.com/doi/full/10.1002/wat2.1416. Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/pure/about/ebr-terms Received: 29 June 2019 Revised: 11 January 2020 Accepted: 28 January 2020 DOI: 10.1002/wat2.1416 FOCUS ARTICLE The role of freshwater bioacoustics in ecological research Jack A. Greenhalgh1 | Martin J. Genner1 | Gareth Jones1 | Camille Desjonquères2 1School of Biological Sciences, University of Bristol, Bristol, UK Abstract 2Terrestrial Ecology Group, Universidad Conventional methodologies used to estimate biodiversity in freshwater ecosys- Autónoma de Madrid, Ciudad tems can be nonselective and invasive, sometimes leading to capture and poten- Universitaria de Cantoblanco, Madrid, tial injury of vulnerable species. Therefore, interest in noninvasive surveying Spain techniques is growing among freshwater ecologists. Passive acoustic monitor- Correspondence ing, the noninvasive recording of environmental sounds, has been shown to Jack A. -

The Distribution Of' Two Species Of' Cenocor/Xa in I~Land Saline Lakes of Bhitisi-I Columbia
:I. F>'T" .llfll.. Su ... Rill '!'. C ,,, . I I ~ lllIi\ , fif) (I%~), Auo. 1, 1969 THE DISTRIBUTION OF' TWO SPECIES OF' CENOCOR/XA IN I~LAND SALINE LAKES OF BHITISI-I COLUMBIA By G . G. E. SCUDDER I ABSTRACT The distribution of Cenocorixa bifida (Hung. ) and C. expleta (U hler) in British Columbia is summarized. The distribution pattern in a series of inland saline lakes in the central interior of the Province is described. All water bodies are in the fli ght range of the two species, and seem to be colonized by them at random. However, C. expleta occ urs and breeds only in saline waters, whereas C. bifida lives and breeds in fresh and moderatelY' saline environments. C. expleta has be en found only in waters with a co nductivity between 3,900 and 29,000 mi~romho s / cm. (at 25°C) : C. bifida occurs only in waters with a conductivity between 20 and 20 ,000 micromhos. cm. The distribution appears to be correlated with sa linity and not with other features of the environment such as area of wa ter body, mean depth, maximum depth, etc. Seven species of Cenocorixa are ity as possible, after the initial survey recorded fro m British Columbia indicated that this was desirable. (Lansbury, 1960), but little is known Those selected were chosen so that about their dis tribution, abundance many other parameters of the en and biology. A comparative study has vironment were alike. Thus all water been started on two of the species C. bodies were situated in the same gen bi/ida (Hung.) and C. -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

Marine Insects
UC San Diego Scripps Institution of Oceanography Technical Report Title Marine Insects Permalink https://escholarship.org/uc/item/1pm1485b Author Cheng, Lanna Publication Date 1976 eScholarship.org Powered by the California Digital Library University of California Marine Insects Edited by LannaCheng Scripps Institution of Oceanography, University of California, La Jolla, Calif. 92093, U.S.A. NORTH-HOLLANDPUBLISHINGCOMPANAY, AMSTERDAM- OXFORD AMERICANELSEVIERPUBLISHINGCOMPANY , NEWYORK © North-Holland Publishing Company - 1976 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise,without the prior permission of the copyright owner. North-Holland ISBN: 0 7204 0581 5 American Elsevier ISBN: 0444 11213 8 PUBLISHERS: NORTH-HOLLAND PUBLISHING COMPANY - AMSTERDAM NORTH-HOLLAND PUBLISHING COMPANY LTD. - OXFORD SOLEDISTRIBUTORSFORTHEU.S.A.ANDCANADA: AMERICAN ELSEVIER PUBLISHING COMPANY, INC . 52 VANDERBILT AVENUE, NEW YORK, N.Y. 10017 Library of Congress Cataloging in Publication Data Main entry under title: Marine insects. Includes indexes. 1. Insects, Marine. I. Cheng, Lanna. QL463.M25 595.700902 76-17123 ISBN 0-444-11213-8 Preface In a book of this kind, it would be difficult to achieve a uniform treatment for each of the groups of insects discussed. The contents of each chapter generally reflect the special interests of the contributors. Some have presented a detailed taxonomic review of the families concerned; some have referred the readers to standard taxonomic works, in view of the breadth and complexity of the subject concerned, and have concentrated on ecological or physiological aspects; others have chosen to review insects of a specific set of habitats. -

Callicorixa Vulnerata)
Evolution, 55(4), 2001, pp. 712±720 TARSAL ASYMMETRY, NUTRITIONAL CONDITION, AND SURVIVAL IN WATER BOATMEN (CALLICORIXA VULNERATA) P. NOSIL1,2 AND T. E. REIMCHEN1,3 1Department of Biology, University of Victoria, P.O. Box 3020, Victoria, British Columbia V8W 3N5, Canada 3E-mail: [email protected] Abstract. Fluctuating asymmetry (FA) has been used as a measure of developmental stability and may indicate individual phenotypic or genotypic quality. Using water boatmen (Callicorixa vulnerata) from a natural population, we examined the relationship between tarsal FA (tarsal spine number, tarsal length) and indices of body condition in two habitats. We used body weight and residual body weight (controlling for body length) as indices of condition because experimental food deprivation in water boatmen led to a reduction in each. We detected a negative relationship between FA and both indices of condition in two ecologically distinct pond habitats. We predicted this association was due to a negative relationship between FA and competitive feeding ability. Consequently, we examined associations between survival time and tarsal FA in C. vulnerata under resource-limited laboratory conditions. Univariate analyses revealed a negative correlation between survival and tarsal FA in each trait. Inclusion of survival time, body length, gender, tarsal spine number, tarsal length, and measures of FA into multivariate analyses revealed a negative correlation between survival and FA. Individuals with the greatest survival had higher nutritional condition than individuals that succumbed early in the experiment. Asymmetric individuals may suffer a foraging handicap as a result of the use of tarsi in feeding or they may be of poor genetic quality. -

CBD Fifth National Report
CONVENTION ON CONVENTION ON BIOLOGICAL DIVERSITY BIOLOGICAL DIVERSITY THE 5TH NATIONAL REPORT OF MONGOLIA biolohJA JJa folea YeehcO beiide& oa KnWWn}A. T HE CONVENTION ON BIOLOGI 5 T H N A T IO N AL R EPO RT C AL DIVERSITY OF M O N GOLIA MINISTRY OF ENVIRONMENT AND GREEN DEVELOPMENT STEPPE FORWARD PROGRAMME, Government building II, BIOLOGY DEPARTMENT, United Nation’s street 5/2, NATIONAL UNIVERSITY OF MONGOLIA TH Chingeltei District, Ulaanbaatar 15160, NUM, Building-2, Ulaanbaatar, Mongolia THE 5 NATIONAL REPORT OF Mongolia P.O.Box 537, Ulaanbaatar 210646A, Tel: 976-51-266197 Ulaanbaatar, Mongolia E-mail: [email protected] Tel: 976-99180148; 976-88305909; 976-88083058 MONGOLIA E-mail: [email protected]; [email protected]; [email protected] Designed by Mongolica Publishing 2014 Ulaanbaatar, Mongolia. 2014 CONVENTION ON BIOLOGICAL DIVERSITY CONVENTION ON BIOLOGICAL DIVERSITY FINANCED BY: MINISTRY OF ENVIRONMENT AND GREEN DEVELOPMENT CONVENTION ON BIOLOGICAL DIVERSITY-MONGOLIA GLOBAL ENVIRONMENT FACILITY UNITED NATIONS ENVIRONMENTAL PROGRAM CONVENTION ON BIOLOGICAL DIVERSITY THE 5TH NATIONAL REPORT OF MONGOLIA REPORT COMPILERS: COMPILED BY: S. GOMBOBAATAR STEPPE FORWARD PROGRAMME, NUM S. MYAGMARSUREN N. CONABOY М. Мunkhjargal TAXON COMPILERS: PLANT: B. OYUNTSETSEG, M. URGAMAL INVERTEBRATE: S. GANTIGMAA Fish, aMphibian, reptile: kh. Тerbish BIRD: S. GOMBOBAATAR MAMMAL: S. SHAR CONTRIBUTIONS FROM: EDITORS: NATIONAL UNIVERSITY OF MONGOLIA INSTITUTE OF BIOLOGY, MONGOLIAN ACADEMY OF SCIENCES D. BATBOLD MONGOLIAN ORNITHOLOGICAL SOCIETY -

Beiträge Zur Bayerischen Entomofaunistik 13: 67–207
Beiträge zur bayerischen Entomofaunistik 13:67–207, Bamberg (2014), ISSN 1430-015X Grundlegende Untersuchungen zur vielfältigen Insektenfauna im Tiergarten Nürnberg unter besonderer Betonung der Hymenoptera Auswertung von Malaisefallenfängen in den Jahren 1989 und 1990 von Klaus von der Dunk & Manfred Kraus Inhaltsverzeichnis 1. Einleitung 68 2. Untersuchungsgebiet 68 3. Methodik 69 3.1. Planung 69 3.2. Malaisefallen (MF) im Tiergarten 1989, mit Gelbschalen (GS) und Handfänge 69 3.3. Beschreibung der Fallenstandorte 70 3.4. Malaisefallen, Gelbschalen und Handfänge 1990 71 4. Darstellung der Untersuchungsergebnisse 71 4.1. Die Tabellen 71 4.2. Umfang der Untersuchungen 73 4.3. Grenzen der Interpretation von Fallenfängen 73 5. Untersuchungsergebnisse 74 5.1. Hymenoptera 74 5.1.1. Hymenoptera – Symphyta (Blattwespen) 74 5.1.1.1. Tabelle Symphyta 74 5.1.1.2. Tabellen Leerungstermine der Malaisefallen und Gelbschalen und Blattwespenanzahl 78 5.1.1.3. Symphyta 79 5.1.2. Hymenoptera – Terebrantia 87 5.1.2.1. Tabelle Terebrantia 87 5.1.2.2. Tabelle Ichneumonidae (det. R. Bauer) mit Ergänzungen 91 5.1.2.3. Terebrantia: Evanoidea bis Chalcididae – Ichneumonidae – Braconidae 100 5.1.2.4. Bauer, R.: Ichneumoniden aus den Fängen in Malaisefallen von Dr. M. Kraus im Tiergarten Nürnberg in den Jahren 1989 und 1990 111 5.1.3. Hymenoptera – Apocrita – Aculeata 117 5.1.3.1. Tabellen: Apidae, Formicidae, Chrysididae, Pompilidae, Vespidae, Sphecidae, Mutillidae, Sapygidae, Tiphiidae 117 5.1.3.2. Apidae, Formicidae, Chrysididae, Pompilidae, Vespidae, Sphecidae, Mutillidae, Sapygidae, Tiphiidae 122 5.1.4. Coleoptera 131 5.1.4.1. Tabelle Coleoptera 131 5.1.4.2. -

(Water Boatmen) Abundance and Contribution to Littoral Zone Fish Forage in Lake Poinsett, South Dakota
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Electronic Theses and Dissertations 1974 Corixidae (Water Boatmen) Abundance and Contribution to Littoral Zone Fish Forage in Lake Poinsett, South Dakota Richard Lee Applegate Follow this and additional works at: https://openprairie.sdstate.edu/etd Part of the Entomology Commons Recommended Citation Applegate, Richard Lee, "Corixidae (Water Boatmen) Abundance and Contribution to Littoral Zone Fish Forage in Lake Poinsett, South Dakota" (1974). Electronic Theses and Dissertations. 5522. https://openprairie.sdstate.edu/etd/5522 This Dissertation - Open Access is brought to you for free and open access by Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. CORIXIDAE (WATER BOATMEN) ABUNDANCE AND CONTRIBUTION TO LITTORAL ZONE FISH FORAGE IN LAKE POINSETT, SOUTH DAKOTA BY RICHARD LEE APPLEGATE A thesis submitted in partial fulfillment of the requirements for the degree Doctor of Philosophy, Major in Entomology, South Dakota State University 1974 SOUTH DAKOTA STATE UNIVERSITY LIBRA�� CORIXIDAE (WATER BOATMEN) ABUNDANCE AND CONTRIBUTION TO LITTORAL ZONE FISH FORAGE IN LAKE POINSETT, SOUTH DAKOTA This thesis is approved as a creditable and independent investigation by a candidate for the degree, Doctor of Philosophy, and is acceptable as meeting the thesis requirements for this degree. Acceptance of this thesis does not imply that the conclu sions reached by the candidate are necessarily the conclusions of the major department. -

(Tropocorixa) Jensenhaarupi (Hemiptera: Heteroptera: Corixidae: Corixini), with Ecological Notes Revista Mexicana De Biodiversidad, Vol
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Melo, María Cecilia; Scheibler, Erica Elizabeth Description of the immature stages ofSigara (Tropocorixa) jensenhaarupi (Hemiptera: Heteroptera: Corixidae: Corixini), with ecological notes Revista Mexicana de Biodiversidad, vol. 82, núm. 1, marzo, 2011, pp. 117-130 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42520745011 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Biodiversidad 82: 117-130, 2011 Description of the immature stages of Sigara (Tropocorixa) jensenhaarupi (Hemiptera: Heteroptera: Corixidae: Corixini), with ecological notes Descripción de los estadios larvales de Sigara (Tropocorixa) jensenhaarupi (Heteroptera: Corixidae), con notas acerca de su ecología María Cecilia Melo1* and Erica Elizabeth Scheibler2 1Departamento Sistemática, Instituto de Limnología “R.A. Ringuelet” (ILPLA) (CCT La Plata CONICET- UNLP), C.C. 712, 1900 La Plata, Argentina. 2Laboratorio de Entomología, Instituto Argentino de Investigaciones de las Zonas Áridas (IADIZA, CCT CONICET, Mendoza), C.C. 507, 5500 Men- doza, Argentina. *Correspondent: [email protected] Abstract. Sigara (Tropocorixa) jensenhaarupi Jaczewski is the smallest species of the subgenus ranging from 4.2-4.7 mm, and it is characterized by the absence of a strigil, the small and narrow genital capsule with a short hypandrium in males, and the shape of the abdominal tergite VII in females. This species is endemic to the Patagonian subregion (Andean region) in Argentina.