Hemiptera: Heteroptera) from India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spezialpraktikum Aquatische Und Semiaquatische Heteroptera SS 2005
Spezialpraktikum Aquatische und Semiaquatische Heteroptera SS 2005 Hinweise zum Bestimmungsschlüssel Das Skriptum ist für den Privatgebrauch bestimmt. Alle Angaben wurden nach bestem Wissen überprüft, Fehler sind aber nicht auszuschließen. Für Anmerkungen und Korrekturen bin ich sehr dankbar. Alle Abbildungen wurden der Literatur entnommen (siehe Literaturverzeichnis) und sind nicht Maßstabsgetreu, d.h. die Größenverhältnisse sind nicht korrekt wiedergegeben. Die Gesamtverbreitungsangaben wurden aus Aukema & Rieger (1995) entnommen und sind daher nicht aktuell. Sie geben aber einen guten Eindruck von der ungefähren Verbreitung der Arten. Die verwendeten zoogeographischen Angaben sind vorläufig und rein deskriptiv zu verstehen. Die Angabe der Bundesländervorkommen in Österreich erfolgt ohne Anspruch auf Vollständigkeit (Rabitsch unveröff., Stand 2005) Wolfgang Rabitsch Wien, Juni 2005 Version 1.1 (Juli 2005) Bestimmungsschlüssel der GERROMORPHA Österreichs Bestimmungsschlüssel der GERROMORPHA Österreichs (verändert nach Andersen 1993, 1995, 1996, Savage 1989, u.a.) Familien (Abb. 1) 1 Kopf mindestens doppelt so lang wie breit, Augen deutlich vom Vorderrand des Prothorax entfernt Hydrometridae - Kopf weniger als doppelt so lang wie breit, Augen berühren fast den Vorderrand des Prothorax 2 2 Coxae inserieren in der Körpermitte, Antennen 4-gliedrig, meist apter Mesoveliidae - Coxae inserieren lateral am Thorax 3 3 Antennen scheinbar 5-gliedrig, Segment 3-5 dünner als Segment 1-2, Ocellen vorhanden Hebridae - Antennen 4-gliedrig, alle Segmente -

Diversity of Water Bugs in Gujranwala District, Punjab, Pakistan
Journal of Bioresource Management Volume 5 Issue 1 Article 1 Diversity of Water Bugs in Gujranwala District, Punjab, Pakistan Muhammad Shahbaz Chattha Women University Azad Jammu & Kashmir, Bagh (AJK), [email protected] Abu Ul Hassan Faiz Women University of Azad Jammu & Kashmir, Bagh (AJK), [email protected] Arshad Javid University of Veterinary & Animal Sciences, Lahore, [email protected] Irfan Baboo Cholistan University of Veterinary & Animal Sciences, Bahawalpur, [email protected] Inayat Ullah Malik The University of Lakki Marwat, Lakki Marwat, [email protected] Follow this and additional works at: https://corescholar.libraries.wright.edu/jbm Part of the Aquaculture and Fisheries Commons, Biodiversity Commons, Entomology Commons, Terrestrial and Aquatic Ecology Commons, and the Zoology Commons Recommended Citation Chattha, M. S., Faiz, A. H., Javid, A., Baboo, I., & Malik, I. U. (2018). Diversity of Water Bugs in Gujranwala District, Punjab, Pakistan, Journal of Bioresource Management, 5 (1). DOI: https://doi.org/10.35691/JBM.8102.0081 ISSN: 2309-3854 online (Received: May 16, 2019; Accepted: Sep 19, 2019; Published: Jan 1, 2018) This Article is brought to you for free and open access by CORE Scholar. It has been accepted for inclusion in Journal of Bioresource Management by an authorized editor of CORE Scholar. For more information, please contact [email protected]. Diversity of Water Bugs in Gujranwala District, Punjab, Pakistan © Copyrights of all the papers published in Journal of Bioresource Management are with its publisher, Center for Bioresource Research (CBR) Islamabad, Pakistan. This permits anyone to copy, redistribute, remix, transmit and adapt the work for non-commercial purposes provided the original work and source is appropriately cited. -

Metacommunities and Biodiversity Patterns in Mediterranean Temporary Ponds: the Role of Pond Size, Network Connectivity and Dispersal Mode
METACOMMUNITIES AND BIODIVERSITY PATTERNS IN MEDITERRANEAN TEMPORARY PONDS: THE ROLE OF POND SIZE, NETWORK CONNECTIVITY AND DISPERSAL MODE Irene Tornero Pinilla Per citar o enllaçar aquest document: Para citar o enlazar este documento: Use this url to cite or link to this publication: http://www.tdx.cat/handle/10803/670096 http://creativecommons.org/licenses/by-nc/4.0/deed.ca Aquesta obra està subjecta a una llicència Creative Commons Reconeixement- NoComercial Esta obra está bajo una licencia Creative Commons Reconocimiento-NoComercial This work is licensed under a Creative Commons Attribution-NonCommercial licence DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode Irene Tornero Pinilla 2020 DOCTORAL THESIS Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode IRENE TORNERO PINILLA 2020 DOCTORAL PROGRAMME IN WATER SCIENCE AND TECHNOLOGY SUPERVISED BY DR DANI BOIX MASAFRET DR STÉPHANIE GASCÓN GARCIA Thesis submitted in fulfilment of the requirements to obtain the Degree of Doctor at the University of Girona Dr Dani Boix Masafret and Dr Stéphanie Gascón Garcia, from the University of Girona, DECLARE: That the thesis entitled Metacommunities and biodiversity patterns in Mediterranean temporary ponds: the role of pond size, network connectivity and dispersal mode submitted by Irene Tornero Pinilla to obtain a doctoral degree has been completed under our supervision. In witness thereof, we hereby sign this document. Dr Dani Boix Masafret Dr Stéphanie Gascón Garcia Girona, 22nd November 2019 A mi familia Caminante, son tus huellas el camino y nada más; Caminante, no hay camino, se hace camino al andar. -

Position Specificity in the Genus Coreomyces (Laboulbeniomycetes, Ascomycota)
VOLUME 1 JUNE 2018 Fungal Systematics and Evolution PAGES 217–228 doi.org/10.3114/fuse.2018.01.09 Position specificity in the genus Coreomyces (Laboulbeniomycetes, Ascomycota) H. Sundberg1*, Å. Kruys2, J. Bergsten3, S. Ekman2 1Systematic Biology, Department of Organismal Biology, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden 2Museum of Evolution, Uppsala University, Uppsala, Sweden 3Department of Zoology, Swedish Museum of Natural History, Stockholm, Sweden *Corresponding author: [email protected] Key words: Abstract: To study position specificity in the insect-parasitic fungal genus Coreomyces (Laboulbeniaceae, Laboulbeniales), Corixidae we sampled corixid hosts (Corixidae, Heteroptera) in southern Scandinavia. We detected Coreomyces thalli in five different DNA positions on the hosts. Thalli from the various positions grouped in four distinct clusters in the resulting gene trees, distinctly Fungi so in the ITS and LSU of the nuclear ribosomal DNA, less so in the SSU of the nuclear ribosomal DNA and the mitochondrial host-specificity ribosomal DNA. Thalli from the left side of abdomen grouped in a single cluster, and so did thalli from the ventral right side. insect Thalli in the mid-ventral position turned out to be a mix of three clades, while thalli growing dorsally grouped with thalli from phylogeny the left and right abdominal clades. The mid-ventral and dorsal positions were found in male hosts only. The position on the left hemelytron was shared by members from two sister clades. Statistical analyses demonstrate a significant positive correlation between clade and position on the host, but also a weak correlation between host sex and clade membership. These results indicate that sex-of-host specificity may be a non-existent extreme in a continuum, where instead weak preferences for one host sex may turn out to be frequent. -

Table of Contents 2
Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) List of Freshwater Macroinvertebrate Taxa from California and Adjacent States including Standard Taxonomic Effort Levels 1 March 2011 Austin Brady Richards and D. Christopher Rogers Table of Contents 2 1.0 Introduction 4 1.1 Acknowledgments 5 2.0 Standard Taxonomic Effort 5 2.1 Rules for Developing a Standard Taxonomic Effort Document 5 2.2 Changes from the Previous Version 6 2.3 The SAFIT Standard Taxonomic List 6 3.0 Methods and Materials 7 3.1 Habitat information 7 3.2 Geographic Scope 7 3.3 Abbreviations used in the STE List 8 3.4 Life Stage Terminology 8 4.0 Rare, Threatened and Endangered Species 8 5.0 Literature Cited 9 Appendix I. The SAFIT Standard Taxonomic Effort List 10 Phylum Silicea 11 Phylum Cnidaria 12 Phylum Platyhelminthes 14 Phylum Nemertea 15 Phylum Nemata 16 Phylum Nematomorpha 17 Phylum Entoprocta 18 Phylum Ectoprocta 19 Phylum Mollusca 20 Phylum Annelida 32 Class Hirudinea Class Branchiobdella Class Polychaeta Class Oligochaeta Phylum Arthropoda Subphylum Chelicerata, Subclass Acari 35 Subphylum Crustacea 47 Subphylum Hexapoda Class Collembola 69 Class Insecta Order Ephemeroptera 71 Order Odonata 95 Order Plecoptera 112 Order Hemiptera 126 Order Megaloptera 139 Order Neuroptera 141 Order Trichoptera 143 Order Lepidoptera 165 2 Order Coleoptera 167 Order Diptera 219 3 1.0 Introduction The Southwest Association of Freshwater Invertebrate Taxonomists (SAFIT) is charged through its charter to develop standardized levels for the taxonomic identification of aquatic macroinvertebrates in support of bioassessment. This document defines the standard levels of taxonomic effort (STE) for bioassessment data compatible with the Surface Water Ambient Monitoring Program (SWAMP) bioassessment protocols (Ode, 2007) or similar procedures. -

UBC 1978 A6 7 C35.Pdf
THE INFLUENCE OF TEMPERATURE AND SALINITY ON THE CUTICULAR PERMEABILITY OF SOME CORIXIDAE by SYDNEY GRAHAM CANNINGS B.Sc, University of British Columbia, 1975 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES (Department of Zoology) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA November, 19 77 © Sydney Graham Cannings, 1977 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of ZOOLOGY The University of British Columbia 2075 Wesbrook Place Vancouver, Canada V6T 1W5 Date November 14, 1977 ABSTRACT Most terrestrial, and many aquatic insects are made waterproof by a layer of lipid in or on the epicuticle. At a specific temperature, which is determined by their composition, these lipids undergo a phase .transition which markedly increases the permeability of the integument. The major purpose of this study was to assess the possibility that epicutic.ular wax transition could differ• entially affect the distribution of four species of water boatmen: Cenocorixa bifida hungerfordi Lansbury, Ceno- corixa expleta (Uhler), Cenocorixa blaisdelli. (Hunger- ford) , and Callicorixa vulnerata (Uhler). -

Synopsis of the Heteroptera Or True Bugs of the Galapagos Islands
Synopsis of the Heteroptera or True Bugs of the Galapagos Islands ' 4k. RICHARD C. JROESCHNE,RD SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 407 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Seznam Vodních Druhů Ploštic
SEZNAM VODNÍCH DRUH Ů PLOŠTIC (HETEROPTERA: NEPOMORPHA, GERROMORPHA) ČR PETR KMENT NEPOMORPHA Popov, 1968 NEPOIDEA Latreille, 1802 NEPIDAE Latreille, 1802 Nepinae Latreille, 1802 Nepini Latreille, 1802 Nepa cinerea Linnaeus, 1758 BM Ranatrinae Douglas & Scott, 1865 Ranatrini Douglas & Scott, 1865 Ranatra (Ranatra) linearis (Linnaeus, 1758) BM CORIXOIDEA Leach, 1815 CORIXIDAE Leach, 1815 Micronectinae Jaczewski, 1924 [Micronecta (Dichaetonecta) pusilla (Horváth, 1895)] Micronceta (Dichaetonecta) scholtzi (Fieber, 1860) BM Micronecta (Micronecta) griseola Horváth, 1899 BM Micronecta (Micronecta) minutissima (Linnaeus, 1758) BM Micronecta (Micronecta) poweri poweri (Douglas & Scott, 1869) BM Cymatiainae Walton, 1940 Cymatia bonsdorffii (C. R. Sahlberg, 1819) BM Cymatia coleoptrata (Fabricius, 1777) BM Cymatia rogenhoferi (Fieber, 1864) BM Corixinae Leach, 1815 Glaenocorisini Hungerford, 1948 Glaenocorisa propinqua (Fieber, 1860) BM Corixini Leach, 1815 Arctocorisa carinata carinata (C. R. Sahlberg, 1819) B Arctocorisa germari (Fieber, 1848) B Callicorixa praeusta praeusta (Fieber, 1848) BM Corixa affinis Leach, 1817 M Corixa dentipes Thomson, 1869 BM Corixa punctata (Illiger, 1807) BM Hesperocorixa castanea (Thomson, 1869) B Hesperocorixa linnaei (Fieber, 1848) BM Hesperocorixa moesta (Fieber, 1848) B Hesperocorixa sahlbergi (Fieber, 1848) BM Paracorixa concinna concinna (Fieber, 1848) BM Sigara (Halicorixa) stagnalis stagnalis (Leach, 1817) ?B Sigara (Microsigara) hellensii (C. R. Sahlberg, 1819) BM Sigara (Pseudovermicorixa) nigrolineata -
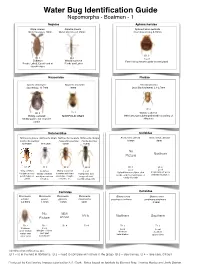
Water Bug ID Guide
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

Buglife Ditches Report Vol1
The ecological status of ditch systems An investigation into the current status of the aquatic invertebrate and plant communities of grazing marsh ditch systems in England and Wales Technical Report Volume 1 Summary of methods and major findings C.M. Drake N.F Stewart M.A. Palmer V.L. Kindemba September 2010 Buglife – The Invertebrate Conservation Trust 1 Little whirlpool ram’s-horn snail ( Anisus vorticulus ) © Roger Key This report should be cited as: Drake, C.M, Stewart, N.F., Palmer, M.A. & Kindemba, V. L. (2010) The ecological status of ditch systems: an investigation into the current status of the aquatic invertebrate and plant communities of grazing marsh ditch systems in England and Wales. Technical Report. Buglife – The Invertebrate Conservation Trust, Peterborough. ISBN: 1-904878-98-8 2 Contents Volume 1 Acknowledgements 5 Executive summary 6 1 Introduction 8 1.1 The national context 8 1.2 Previous relevant studies 8 1.3 The core project 9 1.4 Companion projects 10 2 Overview of methods 12 2.1 Site selection 12 2.2 Survey coverage 14 2.3 Field survey methods 17 2.4 Data storage 17 2.5 Classification and evaluation techniques 19 2.6 Repeat sampling of ditches in Somerset 19 2.7 Investigation of change over time 20 3 Botanical classification of ditches 21 3.1 Methods 21 3.2 Results 22 3.3 Explanatory environmental variables and vegetation characteristics 26 3.4 Comparison with previous ditch vegetation classifications 30 3.5 Affinities with the National Vegetation Classification 32 Botanical classification of ditches: key points -

Ancient Rapid Radiations of Insects: Challenges for Phylogenetic Analysis
ANRV330-EN53-23 ARI 2 November 2007 18:40 Ancient Rapid Radiations of Insects: Challenges for Phylogenetic Analysis James B. Whitfield1 and Karl M. Kjer2 1Department of Entomology, University of Illinois, Urbana, Illinois 61821; email: jwhitfi[email protected] 2Department of Ecology, Evolution and Natural Resources, Rutgers University, New Brunswick, New Jersey 08901; email: [email protected] Annu. Rev. Entomol. 2008. 53:449–72 Key Words First published online as a Review in Advance on diversification, molecular evolution, Palaeoptera, Orthopteroidea, September 17, 2007 fossils The Annual Review of Entomology is online at ento.annualreviews.org Abstract by UNIVERSITY OF ILLINOIS on 12/18/07. For personal use only. This article’s doi: Phylogenies of major groups of insects based on both morphological 10.1146/annurev.ento.53.103106.093304 and molecular data have sometimes been contentious, often lacking Copyright c 2008 by Annual Reviews. the data to distinguish between alternative views of relationships. Annu. Rev. Entomol. 2008.53:449-472. Downloaded from arjournals.annualreviews.org All rights reserved This paucity of data is often due to real biological and historical 0066-4170/08/0107-0449$20.00 causes, such as shortness of time spans between divergences for evo- lution to occur and long time spans after divergences for subsequent evolutionary changes to obscure the earlier ones. Another reason for difficulty in resolving some of the relationships using molecu- lar data is the limited spectrum of genes so far developed for phy- logeny estimation. For this latter issue, there is cause for current optimism owing to rapid increases in our knowledge of comparative genomics. -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA).