How to Sustain Healthcare Transformations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Analyst Report
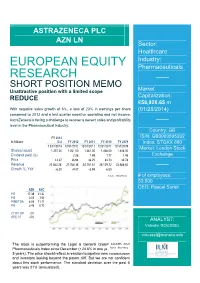
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Affymetrix and the "DNA Chip" Revolution
taken pride in placing in the top 10 among the The Evolution of Astra best companies to work for in a number of ∗ magazine surveys, from Fortune to Working Merck Inc. in 1998 Mother. Merck & Co. sales grew from $9.6 billion in 1992 to $23 billion by the end of "We plan to revolutionize the pharmaceutical industry by 19972, topping the world drug sales league in being the best at linking patients and products.” Wayne Yetter, President of Astra Merck Inc. both years. In fact, Merck has seen its global market share jump one-third in the 1992-1998 period to edge Glaxo Wellcome in 1997 with a Section I. Background 3 4.6% world market share. In contrast to the In 1982, Merck & Co. and Astra AB signed an strategy of other pharmaceutical companies such agreement by which products resulting from as Glaxo, this growth was largely fueled by Astra’s research pipeline would be taken through internal R&D efforts as opposed to mergers and clinical trials and registration, to be ultimately acquisitions. marketed and sold by Merck in the United States. The initial terms of the contract specified Another major strategic move made to enhance that Astra’s products would be transferred to its competitive position was the acquisition of Merck’s development and marketing Medco Containment Services Inc. in 1993 organizations, with Astra receiving royalties on (renamed Merck-Medco Managed Care). the sales of the Astra drugs.1 Merck-Medco provides pharmaceutical benefit services in the United States to control The initial cooperation and relationship between prescription drug benefits cost. -

Astrazeneca 17Th January 2013 Attractive Risk-Reward As New CEO Comes in Healthcare Fair Value 3440P Vs
INDEPENDENT RESEARCH AstraZeneca 17th January 2013 Attractive risk-reward as new CEO comes in Healthcare Fair Value 3440p vs. 2860p (price 3,042p) BUY vs. NEUTRAL Bloomberg AZN LN Among the big names in the pharmaceutical industry, AstraZeneca is Reuters AZN.L obviously one of the few which carries in principle one of the most 12-month High / Low (p) 3,112 / 2,591 significant upsides considering its current valuation, provided the new Market capitalisation (GBPm) 37,921 Enterprise Value (BG estimates GBPm) 39,062 CEO, Pascal Soriot, is able not only to present a comprehensive and Avg. 6m daily volume ('000 shares) 2,247 clear strategy but also to package his speech in an attractive manner Free Float 100% for the investment community to jump in as early as the beginning of 3y EPS CAGR [2012-2015] -1.2% Gearing (12/11) 8% 2013. Dividend yields (12/12e) 5.72% On 31 January 2013, Pascal Soriot is expected to present his initial YE December 12/11 12/12e 12/13e 12/14e thoughts about how to drive AstraZeneca forward and which strategy to Revenue (USDm) 33,591 28,081 27,524 27,251 EBIT(USDm) 12,795 7,921 7,818 8,334 implement. Since he took over as CEO on 1 October 2012, he has spent Basic EPS (USD) 7.33 4.81 4.58 5.04 much time meeting people within the group and also key shareholders to Core EPS (USD) 7.72 6.71 6.13 6.33 EV/Sales 1.9x 2.2x 2.2x 2.0x make the best possible assessment of the situation and to hear their EV/EBITDA 4.1x 6.0x 5.6x 5.0x expectations and hopes before presenting a roadmap. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

1999 Annual Report and Form 20-F CONTENTS
1999 Annual Report and Form 20-F CONTENTS Key Achievements in 1999 1 Financial Highlights 2 Shareholder Highlights 5 Chairman’s Statement 6 Chief Executive’s Review 7 Operational Review 8 Safety, Health and Environment 32 In April 1999, Astra AB and Zeneca Group PLC People and Community 34 merged to form AstraZeneca, one of the world’s Financial Review 35 leading pharmaceutical and agrochemical Board of Directors and Officers companies, which provides innovative, effective of the Company 48 products to improve health, nutrition and quality Directors’ Report 49 of life worldwide. The company is research and Financial Statements 55 technology intensive, with extensive international Financial statements and notes development and marketing skills. Its healthcare relating to the financial statements 55 business is strategically focused on seven major Principal subsidiaries, joint ventures therapeutic areas: gastrointestinal, oncology, pain and associates 118 control and anaesthesia, cardiovascular, central Additional information for US investors 120 nervous system, respiratory and infection. Zeneca Group Financial Record 129 Agrochemicals provides crop protection products Shareholder Information 131 designed to improve crop yields and food quality. Exchange rates 138 Definitions 139 Glossary of Terms 140 Cross Reference to Form 20-F IBC Cautionary statement regarding forward-looking statements In order to utilise the ‘Safe Harbor’ provisions of the United States Private Securities Litigation Reform Act of 1995, AstraZeneca is providing the following cautionary statement. This Annual Report and Form 20-F 1999 contains forward-looking statements with respect to the financial condition, results of operations and businesses of AstraZeneca. By their nature, forward-looking statements and forecasts involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future. -

Astrazeneca Annual Report 2006
AND FORM 20-F ANNUAL INFORMATION REPORT 2006 CONTENTS 2006 IN BRIEF 1 FINANCIAL STATEMENTS 19. Reserves 120 CHAIRMAN’S STATEMENT 2 Preparation of the Financial Statements 20. Minority interests 121 CHIEF EXECUTIVE OFFICER’S REVIEW 3 and Directors’ Responsibilities 96 21. Dividends to shareholders 121 FINANCIAL HIGHLIGHTS 6 Directors’ Responsibilities for, and Report 22. Acquisitions of on, Internal Control over Financial DIRECTORS’ REPORT business operations 121 Reporting 96 Business review 8 23. Disposal of business operations 123 Auditors’ Reports on the Financial 24. Post-retirement benefi ts 123 > Business environment 9 Statements and on Internal Control over > Strategy 11 Financial Reporting (Sarbanes-Oxley Act 25. Employee costs and share option plans for employees 128 > Our resources, skills Section 404) 97 and capabilities 12 Independent Auditors’ Report to the 26. Commitments and contingent liabilities 133 > Measuring performance 15 Members of AstraZeneca PLC (Group) 97 27. Leases 146 > Therapy area review Consolidated Income Statement 98 28. Statutory and other information 146 – Cardiovascular medicines 16 Consolidated Statement of Recognised Income and Expense 98 29. Share capital of parent – Gastrointestinal medicines 20 Consolidated Balance Sheet 99 company 147 – Neuroscience medicines 23 Consolidated Cash Flow Statement 100 Principal Subsidiaries 148 – Oncology medicines 26 Accounting Policies (Group) 101 Additional Information for US Investors 149 – Respiratory and Independent Auditors’ Infl ammation medicines 29 Notes to the Financial Statements (Group) Report to the Members of – Infection medicines 32 AstraZeneca PLC (Company) 157 1. Operating profi t 104 > Geographic review 33 Company Balance Sheet 158 2. Profi t on sale of interest > Research and development 37 in joint venture 104 Accounting Policies (Company) 159 > Development pipeline table 40 3. -

Astrazeneca: the First Abuse Case in the Pharmaceutical Sector Niklas FAGERLUND and Søren Bo RASMUSSEN, Directorate-General Competition, Unit B-2
Antitrust AstraZeneca: the first abuse case in the pharmaceutical sector Niklas FAGERLUND and Søren Bo RASMUSSEN, Directorate-General Competition, unit B-2 1. Introduction by AZ before patent offices in Belgium, Denmark, Germany, the Netherlands, Norway and the United On 15 June 2005 the Commission adopted a deci- Kingdom and before national courts in Germany sion (‘Decision’) fining the Swedish company and Norway. AstraZeneca AB and the UK company Astra- Zeneca Plc (together ‘AZ’) 60 million euros due to The misleading information was provided by AZ their infringements of Article 82 of the EC Treaty in the context of its two rounds of applications and Article 54 of the EEA Agreement. (in June 1993 and December 1994) to several pat- ent offices within the EEA for extra protection for The infringements involve misuses by AZ of public omeprazole (the active substance in AZ’s product procedures and regulations in a number of EEA Losec) in the form of so-called supplementary states aimed at excluding generic firms and paral- protection certificates (SPCs). Under the SPC Reg- lel traders from competing against AZ’s anti-ulcer ulation the basic patent protection for active sub- product Losec. stances in medicines can be extended by a maxi- In 1979, Astra AB (currently AstraZeneca AB), a mum of five years. Swedish research based company, had filed pat- The Decision raises no objections to AZ’s incorrect ent applications in Europe in respect of omepra- interpretation of the relevant legislation. Therefore, zole (the active substance in Losec). Losec’s basic the proceedings and outcome in Hässle AB v. -

Astrazeneca Notice of AGM 2007
NOTICE OF ANNUALAND SUMMARY GENERAL MEETINGFINANCIAL STATEMENT 2007 AND SHAREHOLDERS’ cIRCULAR WorldReginfo - 94cdbe6b-e6ef-4290-869e-95f1d4a336fd WorldReginfo - 94cdbe6b-e6ef-4290-869e-95f1d4a336fd LETTER FROM THE CHAIRMAN 1 LETTER FROM THE CHAIRMAN THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE AttENTION. IF YOU ARE or incurring because of the Political Parties, IN ANY DOUBT ABOUT ITS CONTENTS OR WHAT ACTION YOU SHOULD TAKE, YOU SHOULD Elections and Referendums Act 2000 in the CONSULT YOUR INDEPENDENT FINANCIAL ADVISER. IF YOU HAVE SOLD OR TRANSFERRED ALL UK (“the Act”). OF YOUR ASTRAZENECA ORDINARY SHARES YOU SHOULD SEND THIS DOCUMENT AND THE ACCOMPANYING DOCUMENTS TO THE PURCHASER OR TRANSFEREE OR TO THE STOCKBROKER, The Company has no intention of changing its BANK OR OTHER AGENT THROUGH WHOM THE SALE OR TRANSFER WAS EFFECTED FOR current practice of not making donations to TRANSMISSION TO THE PURCHASER OR TRANSFEREE. political parties in the EU and it will not do so without the specific endorsement of its shareholders. However, the Act defines DEAR SHAREHOLDER Senior Independent Director. Sir Peter was ‘political organisation’ widely so as to include, On behalf of the Board of AstraZeneca PLC, first appointed to the Board in January 1995. amongst other things, an organisation that I enclose various documents concerning your carries on activities that are capable of being shareholding in the Company. These are: On behalf of the whole Board, I would like reasonably regarded as intended to influence to express our gratitude to Erna and Sir public support for a political party in any EU 1 A Shareholders’ Circular incorporating Peter for their considerable contribution to member state or to influence voters in relation the formal Notice of the Annual General AstraZeneca’s success and wish them both to any referendum in any EU member state. -

Annual Report and Form 20-F Information 2003 AZ AR Innercovs.Qxd 18/2/04 3:58 PM Page 1
AZ_AR_OuterCovs.qxd 18/2/04 3:59 PM Page 1 AstraZeneca Annual Report and Form 20-F Information 2003 Annual Report and Form 20-F Information 2003 AZ_AR_InnerCovs.qxd 18/2/04 3:58 PM Page 1 AstraZeneca Annual Report and Contents Form 20-F Information 2003 Contents Cross Reference to The information in this document that is r Key Achievements 01 Financial Statements 21. Reserves 86 with the Securities and Exchange Comm Chairman’s Statement 02 Preparation of the Financial Statements 22. Net cash inflow from into any filings by AstraZeneca under the Global Market Overview 03 and Directors’ Responsibilities 60 trading operations 87 such major headings, including subhead Chief Executive’s Review 04 Basis of Consolidation and Presentation 23. Cash flows related Graphs are not included unless specifica Financial Highlights 05 of Financial Information 60 to exceptional items 87 SEC passed comment upon the accurac Board of Directors 06 Independent Auditor’s Report to the 24. Acquisitions of subsidiaries and information and may be updated from tim Members of AstraZeneca PLC 61 purchases of minority interests 87 Strategy 08 Group Profit and Loss Account 62 25. Disposals of business operations 88 Operational Review 09 Group Statement of Total Recognised 26. Reconciliation of net cash flow Cardiovascular 10 Item Gains and Losses 62 to movement in net funds 88 3 Key Information Gastrointestinal 12 Group Balance Sheet 64 27. Analysis of net funds 89 Neuroscience 14 A. Selected financial data Statement of Group Cash Flow 65 28. Financing 89 Financial Highlights Oncology 16 Accounting Policies 66 29. Post-retirement benefits 90 Group Financial Record Respiratory and Inflammation 18 Notes to the Financial Statements 30. -

NOBEL MOLECULAR Frontiers
NOBEL WORKSHOP & MOLECULAR FRONTIERS SYMPOSIUM Nobel Workshop & Molecular Frontiers Symposium organized by: An Amazing Week at Chalmers May 4th-8th 2015 RunAn Conference Hall, Chalmers University of Technology Chalmersplatsen 1, Gothenburg, Sweden !"#$%&'(") )*!%)!") Welcome!( The!Nobel!Workshop!and!Molecular!Frontiers!Symposium!in!Gothenburg!are!spanning!over! widely!distant!horizons!of!the!molecular!paradigm.!From!addressing!intriguing!questions!of! life! itself,! how! it! once! began! and! how! molecules! like! cogwheels! work! together! in! the! complex! machinery! of! the! cell! E! to! various! practical! applications! of! molecules! in! novel! materials!and!in!energy!research;!from!how!biology!is!exploiting!its!molecules!for!driving!the! various! processes! of! life,! to! how! insight! into! the! fundamentals! of! photophysics! and! photochemistry!of!molecules!may!give!us!clues!about!solar!energy!and!tools!by!which!we! may!tame!it!for!the!benefit!of!all!of!us,!and!our!environment.!! ! ! Science!is!sometimes!artificially!divided!into!“fundamental”!and!”applied”!but!these! terms!are!irrelevant!because!research!is!judged!to!be!groundbreaking!by!the!consequences! it!may!have.!Any!groundbreaking!fundamental!result!has!sooner!or!later!consequences!in! applications,!and!the!limits!are!often!only!drawn!by!our!imagination.!! ! ! Science!is!very!much!a!matter!of!communication:!we!not!only!learn!from!each!other! (facts,!ideas!and!concepts),!we!also!need!interactions!for!inspiration!and!as!testing!ground! for!our!ideas.!A!successful!scientific!communication!(publication!or!lecture)!always!requires! -

Annual Report 2012
Registered office and Investor relations Registrar US Depositary 2012 20-F Information Form and Report Annual AstraZeneca AstraZeneca Annual Report corporate headquarters [email protected] Equiniti Limited JPMorgan Chase & Co and Form 20-F Information 2012 AstraZeneca PLC Aspect House PO Box 64504 2 Kingdom Street UK: as above Spencer Road St Paul London W2 6BD Lancing MN 55164-0504 UK US West Sussex BN99 6DA US Tel: +44 (0)20 7604 8000 Investor Relations UK Tel: (toll free in the US) Fax: +44 (0)20 7604 8151 AstraZeneca Pharmaceuticals LP Tel: (freephone in the UK) 888 697 8018 1800 Concord Pike 0800 389 1580 Tel: (outside the US) PO Box 15437 Tel: (outside the UK) +1 (651) 453 2128 Wilmington +44 (0)121 415 7033 [email protected] DE 19850-5437 US Swedish Central Securities Tel: +1 (302) 886 3000 Depository Fax: +1 (302) 886 2972 Euroclear Sweden AB PO Box 191 SE-101 23 Stockholm Sweden Tel: +46 (0)8 402 9000 Delivering value This Annual Report is also available on through innovation our website, astrazeneca.com/ annualreport2012 Important information for readers of this Annual Report AstraZeneca Cautionary statement regarding Innovation is at the core of everything we forward-looking statements Welcome to the AstraZeneca The purpose of this Annual Report is to provide Annual Report and Form information to the members of the Company. The do at AstraZeneca – from our research 20-F Information 2012 Company and its Directors, employees, agents and (Annual Report). You will find advisers do not accept or assume responsibility to into effective new medicines to how we this Annual Report on our any other person to whom this Annual Report is website, astrazeneca.com/ shown or into whose hands it may come and any annualreport2012 such responsibility or liability is expressly disclaimed. -

Nexium (A): Launch Pricing and Dosage Strengths)
1 [maximum 14 days of treatment every four months], Nexium (A): Launch Pricing and dosage strengths). Critics, such as Marcia Angell, place “the blame” [for Nexium’s market dominance and success as a follow on to Prilosec] on AZ’s “audacious plan” to “spin out old products” rather than develop new entities. In contrast, New Yorker columnist and economist Malcolm Gladwell sees it differently. Nexium (esomeprazole) was approved by the FDA in “AstraZeneca was able to do some chemical sleight February 2001 and launched in the US by Astra of hand, spend half a billion on advertising, and get Zeneca (AZ) in June of that year. Esomeprazole is in away with the “reinvention” of its heartburn drug the proton pump inhibitor (PPI) class of drugs, which only because that consensus [what we want from our block the production of acid by the stomach. PPI’s medical system] hasn’t yet been reached. For sellers are primarily indicated for use as treatments of to behave responsibly, buyers must first behave stomach and duodenal ulcers and gastroesophageal intelligently. And if we want to create a system where reflux disease (GERD). AZ launched Nexium just as millions of working and elderly Americans don’t have the patent was expiring on AZ’s largest selling drug, to struggle to pay for prescription drugs that’s also the PPI market leader, Prilosec (omeprazole). up to us. We could find it in our hearts to provide all Americans with adequate health insurance. It is only Nexium has been controversial. Since its launch, AZ by the most spectacular feat of cynicism that our has been accused of making “false and misleading” political system’s moral negligence has become the claims for Nexium’s effectiveness; Tom Scully, then fault of the pharmaceutical industry”.