About Atlantic Capital Markets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Analyst Report
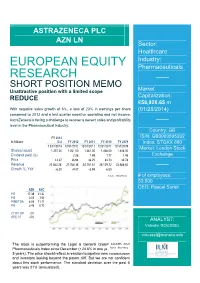
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Nexium Control, Esomeprazole
27 June 2013 EMA/498929/2013 Committee for Medicinal Products for Human Use (CHMP) Assessment report Nexium Control International non-proprietary name: esomeprazole Procedure No. EMEA/H/C/002618 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8613 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Product information Marketing authorisation application Name of the medicinal product: Nexium Control Applicant: AstraZeneca AB Building 411A, Floor 4 S - 151 85 Södertälje SWEDEN Active substance: esomeprazole (as magnesium trihydrate) International Nonproprietary Name/Common Name: esomeprazole Pharmaco-therapeutic group Proton pump inhibitors (ATC Code): (A02BC05) Therapeutic indication: Nexium Control is indicated for the short-term treatment of reflux symptoms (e.g. heartburn and acid regurgitation) in adults. Pharmaceutical form: Gastro-resistant tablet Strength: 20 mg Route of administration: Oral use Packaging: blister (PVC/PVDC) Package sizes: 7 tablets and 14 tablets Assessment report EMA/498929/2013 Page 2/70 Table of contents 1. Background information on the procedure .............................................. 6 1.1. Submission of the dossier ..................................................................................... -

Affymetrix and the "DNA Chip" Revolution
taken pride in placing in the top 10 among the The Evolution of Astra best companies to work for in a number of ∗ magazine surveys, from Fortune to Working Merck Inc. in 1998 Mother. Merck & Co. sales grew from $9.6 billion in 1992 to $23 billion by the end of "We plan to revolutionize the pharmaceutical industry by 19972, topping the world drug sales league in being the best at linking patients and products.” Wayne Yetter, President of Astra Merck Inc. both years. In fact, Merck has seen its global market share jump one-third in the 1992-1998 period to edge Glaxo Wellcome in 1997 with a Section I. Background 3 4.6% world market share. In contrast to the In 1982, Merck & Co. and Astra AB signed an strategy of other pharmaceutical companies such agreement by which products resulting from as Glaxo, this growth was largely fueled by Astra’s research pipeline would be taken through internal R&D efforts as opposed to mergers and clinical trials and registration, to be ultimately acquisitions. marketed and sold by Merck in the United States. The initial terms of the contract specified Another major strategic move made to enhance that Astra’s products would be transferred to its competitive position was the acquisition of Merck’s development and marketing Medco Containment Services Inc. in 1993 organizations, with Astra receiving royalties on (renamed Merck-Medco Managed Care). the sales of the Astra drugs.1 Merck-Medco provides pharmaceutical benefit services in the United States to control The initial cooperation and relationship between prescription drug benefits cost. -

Esomeprazole: a New Proton Pump Inhibitor for NSAID-Associated Peptic Ulcers and Dyspepsia
DRUG PROFILE Esomeprazole: a new proton pump inhibitor for NSAID-associated peptic ulcers and dyspepsia Grace Lai-Hung Wong Ulcer and ulcer symptoms related to the use of non-steroidal anti-inflammatory drugs & Joseph JY Sung† (NSAIDs) and aspirin constitute a major global health issue. Despite various attempts to †Author for correspondence prevent and heal injuries inflicted by NSAIDs and aspirin, acid suppression remains one of The Prince of Wales Hospital, Department of Medicine and the cornerstones in the management of NSAID-associated ulcers. Esomeprazole, the Therapeutics, 9/F, S-optical isomer (enantiomer) of omeprazole, suppresses gastric acid secretion by 30–32 Ngan Shing Road, inhibiting the parietal cell membrane enzyme H+/K+-ATPase. With improved bioavailability, Shatin, NT, Hong Kong SAR due to reduced first-pass metabolism in the liver, esomeprazole promises to be more Tel.: +852 2632 3132 potent in acid suppression in the stomach. Similar to omeprazole, the safety profile of Fax: +852 2645 1699 esomeprazole has been well established. Clinical studies comparing esomeprazole with [email protected] other proton pump inhibitors (PPIs) in the healing of NSAID-related ulcer are few. Recent multicenter randomized studies demonstrated that esomeprazole significantly improves dyspeptic symptoms in patients taking nonselective NSAIDs and specific cyclooxygenase-2 inhibitors. Esomeprazole also protects the stomach from aspirin-induced ulcer bleeding. Safety profiles of esomeprazole appear promising. Non-steroidal anti-inflammatory drugs both basal and stimulated acid secretion. PPIs are (NSAIDs) are the most commonly used medica- most effective against meal-induced gastric acid tions for chronic pain and arthritis due to their secretion [3]. -

Dr. Reddy's – Recall of Ranitidine
Dr. Reddy’s – Recall of ranitidine • On October 23, 2019, Dr. Reddy’s announced the voluntary, consumer-level recall of prescription ranitidine due to potential contamination with N-nitrosodimethylamine (NDMA). • Dr. Reddy’s initially announced retail level recalls for prescription and OTC ranitidine in early October; however, the recall of prescription ranitidine products has been escalated to the consumer level. The recall of OTC ranitidine remains at the retail level. Prescription Ranitidine Capsules Recalled by Dr. Reddy’s Product Description NDC# Ranitidine 150 mg capsules, 60 count bottle 55111-129-60 Ranitidine 150 mg capsules, 500 count bottle 55111-129-05 Ranitidine 300 mg capsules, 30 count bottle 55111-130-30 Ranitidine 300 mg capsules, 100 count bottle 55111-130-01 • Refer to the Dr. Reddy’s announcement for a complete list of the OTC recalled ranitidine products. • Other manufacturers, including Apotex, Perrigo and Sanofi have recently announced retail level recalls of their OTC ranitidine products. • In September, Sandoz issued a consumer level recall of prescription ranitidine products. • These recalls follow a recent FDA statement about NDMA impurities detected in ranitidine medicines. • NDMA is classified as a probable human carcinogen based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables. • Ranitidine is an OTC and prescription drug. Ranitidine is an H2 (histamine-2) blocker, which decreases the amount of acid created by the stomach. OTC ranitidine is approved to prevent and relieve heartburn associated with acid ingestion and sour stomach. • Prescription ranitidine is approved for multiple indications, including treatment and prevention of ulcers of the stomach and intestines and treatment of gastroesophageal reflux disease. -

Label Phase of the Study
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ------------------------WARNINGS AND PRECAUTIONS---------------- NEXIUM safely and effectively. See full prescribing information for • Symptomatic response does not preclude the presence of gastric NEXIUM. malignancy (5.1) • Atrophic gastritis has been noted with long-term omeprazole therapy NEXIUM (esomeprazole magnesium) Delayed-Release Capsules, for Oral (5.2) Use • PPI therapy may be associated with increased risk of Clostridium NEXIUM (esomeprazole magnesium) For Delayed-Release Oral difficile associated diarrhea. (5.3) Suspension • Bone Fracture: Long-term and multiple daily dose PPI therapy may be Initial U.S. Approval: 1989 (omeprazole) associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5.4) -----------------------RECENT MAJOR CHANGES----------------------- • Hypomagnesemia has been reported rarely with prolonged treatment Warnings and Precautions, Clostridium difficile associated 09/2012 with PPIs (5.5) diarrhea (5.3) • Avoid concomitant use of NEXIUM with St John’s Wort or rifampin Warnings and Precautions, Concomitant use of NEXIUM with due to the potential reduction in esomeprazole levels (5.6) (7.3) Methotrexate (5.10) 01/2012 • Interactions with diagnostic investigations for Neuroendocrine Tumors: Increases in intragastric pH may result in hypergastrinemia and -----------------------INDICATIONS AND USAGE----------------------- enterochromaffin-like cell hyperplasia and -

Proton Pump Inhibitors Aciphex
PHARMACY PRE-AUTHORIZATION CRITERIA DRUG (S) Proton Pump Inhibitors Aciphex (Brand name) Dexilant Nexium/esomeprazole (RX Version) Omeprazole-bicarbonate Prevacid (Brand name) Prilosec (Brand name) Protonix (Brand name) Zegerid (RX Version and Brand name) POLICY # 14157 INDICATIONS These agents are approved for the treatment of duodenal ulcers, gastroesophageal reflux disease (GERD), Zollinger Ellison syndrome and H. pylori eradication. CRITERIA DRUG FREEDOM DRUG LIST Step COMMERCIAL DRUG LIST Step Requirements Requirements Aciphex (brand All of these: All of these: name) • Nexium OTC • Nexium OTC • Prilosec OTC , omeprazole, • Prilosec OTC , omeprazole, or Zegerid OTC or Zegerid OTC • Prevacid 24HR OTC/ • Prevacid 24HR OTC/ lansoprazole lansoprazole • Protonix/pantoprazole • Protonix/pantoprazole Dexilant All of these: ALL of these: • Nexium OTC • Nexium OTC • Prilosec OTC , omeprazole, • Prilosec OTC , omeprazole, or Zegerid OTC or Zegerid OTC • Prevacid 24HR OTC/ • Prevacid 24HR OTC/ lansoprazole lansoprazole • Protonix/pantoprazole • Protonix/pantoprazole esomeprazole All of these: All of these: • Nexium OTC • Nexium OTC • Prilosec OTC , omeprazole, • Prilosec OTC , omeprazole, or Zegerid OTC or Zegerid OTC • Prevacid 24HR OTC/ • Prevacid 24HR OTC/ PHARMACY PRE-AUTHORIZATION CRITERIA lansoprazole lansoprazole • Protonix/pantoprazole • Protonix/pantoprazole lansoprazole PA Not Required PA Not Required Nexium (RX All of these: All of these: Version) • Nexium OTC • Nexium OTC • Prilosec OTC , omeprazole, • Prilosec OTC , omeprazole, or -

PHRP March 2015
March 2015; Vol. 25(2):e2521518 doi: http://dx.doi.org/10.17061/phrp2521518 www.phrp.com.au Research Manual versus automated coding of free-text self-reported medication data in the 45 and Up Study: a validation study Danijela Gnjidica,b,i, Sallie-Anne Pearsona,c, Sarah N Hilmerb,d, Jim Basilakise, Andrea L Schaffera, Fiona M Blythb,f,g and Emily Banksg,h, on behalf of the High Risk Prescribing Investigators a Faculty of Pharmacy, University of Sydney, NSW, Australia b Sydney Medical School, University of Sydney, NSW, Australia c Sydney School of Public Health, University of Sydney, NSW, Australia d Royal North Shore Hospital and Kolling Institute of Medical Research, Sydney, NSW, Australia e School of Computing, Engineering and Mathematics, University of Western Sydney, NSW, Australia f Centre for Education and Research on Ageing (CERA), Concord Hospital, Sydney, NSW, Australia g The Sax Institute, Sydney, NSW, Australia h National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT i Corresponding author: [email protected] Article history Abstract Publication date: March 2015 Background: Increasingly, automated methods are being used to code free- Citation: Gnjidic D, Pearson S, Hilmer S, text medication data, but evidence on the validity of these methods is limited. Basilakis J, Schaffer AL, Blyth FM, Banks E. To examine the accuracy of automated coding of previously keyed Manual versus automated coding of free-text Aim: in free-text medication data compared with manual coding of original self-reported medication data in the 45 and Up Study: a validation study. Public Health handwritten free-text responses (the ‘gold standard’). -

Acid-Reducing Medications: Interactions with Harvoni and Epclusa
November 2016 | www.hepatitis.va.gov Acid-Reducing Medications: Interactions with Harvoni® and Epclusa® Sofosbuvir/Velpatasvir = Epclusa® Ledipasvir/Sofosbuvir = Harvoni® • Acid-reducing medications can decrease the effectiveness of Harvoni® and Epclusa® and could prevent successful cure. • Let your provider know if you are taking any acid-reducing medications—either prescribed to you or purchased over the counter. Types of Acid-Reducing What to Do if What to Do if Medications Taking Epclusa® Taking Harvoni® Antacids Separate taking the antacid and Separate taking the antacid and Epclusa® by 4 hours Harvoni® by 4 hours Combinations of aluminum, magnesium, and calcium. Brand names include: Alka-Seltzer® Maalox® Mylanta® Rolaids® TUMS® H2 Blockers Do not take more than the Do not take more than the recommended doses recommended doses Cimetidine (Tagamet®) Famotidine (Pepcid®) May take together with Epclusa® May take together with Harvoni® Nizatidine (Axid®) or 12 hours apart or 12 hours apart Ranitidine (Zantac®) Proton Pump Inhibitors (PPI) It is best to avoid PPIs during treat- It is best to avoid PPIs during ment with Epclusa®. If there are no treatment with Harvoni®. If there Esomeprazole (Nexium®) alternatives, follow these instructions: are no alternatives, follow these Lansoprazole (Prevacid®) instructions: Omeprazole (Prilosec®) Take Epclusa® with food Pantoprazole (Protonix®) Take the proton pump inhibitor 4 Take Harvoni® and the proton Rabeprazole (Aciphex®) hours after taking Epclusa® pump inhibitor at the same time in the morning after fasting overnight Do not take the proton pump (on an empty stomach) inhibitor at the same time as Epclusa® Do not take proton pump Do not take proton pump inhibitors inhibitors at more than the at more than the recommended dose recommended dose U.S. -

Medication Guide Esomeprazole Magnesium
MEDICATION GUIDE ESOMEPRAZOLE MAGNESIUM DELAYED-RELEASE CAPSULES, USP (es″ oh mep′ ra zole mag nee′ zee um) 20 mg and 40 mg Read the Medication Guide that comes with esomeprazole magnesium delayed-release capsules before you start taking esomeprazole magnesium delayed-release capsules and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. What is the most important information I should know about esomeprazole magnesium delayed-release capsules? Esomeprazole magnesium delayed-release capsules may help your acid-related symptoms, but you could still have serious stomach problems. Talk with your doctor. Esomeprazole magnesium delayed-release capsules can cause serious side effects, including: • Diarrhea. Esomeprazole magnesium delayed-release capsules may increase your risk of getting severe diarrhea. This diarrhea may be caused by an infection ( Clostridium difficile) in your intestines. • Call your doctor right away if you have watery stool, stomach pain, and fever that does not go away. • Bone fractures. People who take multiple daily doses of Proton Pump Inhibitor medicines for a long period of time (a year or longer) may have an increased risk of fractures of the hip, wrist, or spine. You should take esomeprazole magnesium delayed-release capsules exactly as prescribed, at the lowest dose possible for your treatment and for the shortest time needed. Talk to your doctor about your risk of bone fracture if you take esomeprazole magnesium delayed-release capsules. • Esomeprazole magnesium delayed-release capsules can have other serious side effects. See “What are the possible side effects of esomeprazole magnesium delayed-release capsules?” What are esomeprazole magnesium delayed-release capsules? Esomeprazole magnesium delayed-release capsules are a prescription medicine called a proton pump inhibitor (PPI). -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

How to Sustain Healthcare Transformations
Voices on transformation A marathon, not a sprint: How to sustain healthcare transformations Voices on transformation is written by experts and practitioners in McKinsey & Company’s Pharmaceuticals and Medical Products Practice. To send comments or request copies of this publication, please email us at [email protected] Editors: Gayane Gyurjyan, Ioana Parsons, Shail Thaker, Jill Willder, Carla Zwaanstra Artwork and design: Afitha de Rijk-Voeten Portrait illustrations: Allan Burch Special thanks to: Lucia Darino, Martin Dewhurst, Claudio Feser, Vincent Forlenza, Jane Griffiths, Judith Hazlewood, Nadine Mansour, Angelika Reich, Pascal Soriot, David Speiser, Kirsten Westhues, André Wyss Copyright © 2016 McKinsey & Company. All rights reserved. This publication is not intended to be used as the basis for trading in the shares of any company or for undertaking any other complex or significant financial transaction without consulting appropriate professional advisers. No part of this publication may be copied or redistributed in any form without the prior written consent of McKinsey & Company. 2 PMP Voices on transformation <Chaper title> Contents Introduction 4 Cracking the code: How successful pharma leaders 7 manage transformations A health check for pharma: Overcoming change fatigue 15 in the pharmaceutical industry Putting science at the heart of renewed purpose 26 An interview with Pascal Soriot, AstraZeneca Transforming a medical devices company into a solutions provider 34 An interview with Vincent Forlenza, Becton Dickinson Refocusing the business around patient outcomes 42 An interview with Jane Griffiths, Janssen EMEA NBS: Creating value across Novartis 48 An interview with André Wyss, Novartis Voices on digital: How pharma can win in a digital world 57 About the authors 66 3 Introduction Over the past decade, the pharmaceutical industry has been struggling to keep up with rapid and dramatic changes in the external environment.