Astrazeneca Plc
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Analyst Report
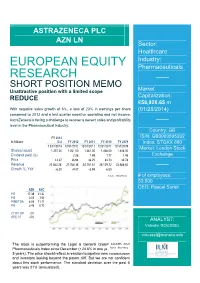
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Nexium Control, Esomeprazole
27 June 2013 EMA/498929/2013 Committee for Medicinal Products for Human Use (CHMP) Assessment report Nexium Control International non-proprietary name: esomeprazole Procedure No. EMEA/H/C/002618 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8613 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Product information Marketing authorisation application Name of the medicinal product: Nexium Control Applicant: AstraZeneca AB Building 411A, Floor 4 S - 151 85 Södertälje SWEDEN Active substance: esomeprazole (as magnesium trihydrate) International Nonproprietary Name/Common Name: esomeprazole Pharmaco-therapeutic group Proton pump inhibitors (ATC Code): (A02BC05) Therapeutic indication: Nexium Control is indicated for the short-term treatment of reflux symptoms (e.g. heartburn and acid regurgitation) in adults. Pharmaceutical form: Gastro-resistant tablet Strength: 20 mg Route of administration: Oral use Packaging: blister (PVC/PVDC) Package sizes: 7 tablets and 14 tablets Assessment report EMA/498929/2013 Page 2/70 Table of contents 1. Background information on the procedure .............................................. 6 1.1. Submission of the dossier ..................................................................................... -

Complementary and Alternative Medicine and the Law 00 Jesson Tovino Fmt 4/21/10 8:30 AM Page Ii 00 Jesson Tovino Fmt 4/21/10 8:30 AM Page Iii
00 jesson tovino fmt 4/21/10 8:30 AM Page i Complementary and Alternative Medicine and the Law 00 jesson tovino fmt 4/21/10 8:30 AM Page ii 00 jesson tovino fmt 4/21/10 8:30 AM Page iii Complementary and Alternative Medicine and the Law Lucinda E. Jesson Hamline University School of Law Stacey A. Tovino William S. Boyd School of Law University of Nevada, Las Vegas Carolina Academic Press Durham, North Carolina 00 jesson tovino fmt 4/21/10 8:30 AM Page iv Copyright © 2010 Lucinda E. Jesson Stacey A. Tovino All Rights Reserved Library of Congress Cataloging-in-Publication Data Jesson, Lucinda E. Complementary and alternative medicine and the law / Lucinda E. Jesson and Stacey A. Tovino. p. cm. ISBN 978-1-59460-767-7 (alk. paper) 1. Medical laws and legislation 2. Alternative medicine--Law and legislation--United States. I. Tovino, Stacey A. II. Title. KF3821.J47 2010 344.7304'1--dc22 2009051502 Carolina Academic Press 700 Kent Street Durham, North Carolina 27701 Telephone (919) 489-7486 Fax (919) 493-5668 www.cap-press.com Printed in the United States of America 00 jesson tovino fmt 4/21/10 8:30 AM Page v Contents Acknowledgments xi Chapter One • Introduction to Complementary and Alternative Medicine 3 I. The Increased Presence of Complementary and Alternative Medicine in the United States 3 A. Evidence of Increased Use and Attention 3 B. Why Are More People Using CAM? 5 II. What Is Complementary and Alternative Medicine? 6 A. Definitions 6 B. Overview of CAM Modalities 9 Osteopathy 9 Chiropractic 11 Massage Therapy 12 Homeopathy 13 Naturopathy 14 Dietary and Herbal Supplements 14 Spirituality 15 Acupuncture 16 Midwifery 17 C. -

Affymetrix and the "DNA Chip" Revolution
taken pride in placing in the top 10 among the The Evolution of Astra best companies to work for in a number of ∗ magazine surveys, from Fortune to Working Merck Inc. in 1998 Mother. Merck & Co. sales grew from $9.6 billion in 1992 to $23 billion by the end of "We plan to revolutionize the pharmaceutical industry by 19972, topping the world drug sales league in being the best at linking patients and products.” Wayne Yetter, President of Astra Merck Inc. both years. In fact, Merck has seen its global market share jump one-third in the 1992-1998 period to edge Glaxo Wellcome in 1997 with a Section I. Background 3 4.6% world market share. In contrast to the In 1982, Merck & Co. and Astra AB signed an strategy of other pharmaceutical companies such agreement by which products resulting from as Glaxo, this growth was largely fueled by Astra’s research pipeline would be taken through internal R&D efforts as opposed to mergers and clinical trials and registration, to be ultimately acquisitions. marketed and sold by Merck in the United States. The initial terms of the contract specified Another major strategic move made to enhance that Astra’s products would be transferred to its competitive position was the acquisition of Merck’s development and marketing Medco Containment Services Inc. in 1993 organizations, with Astra receiving royalties on (renamed Merck-Medco Managed Care). the sales of the Astra drugs.1 Merck-Medco provides pharmaceutical benefit services in the United States to control The initial cooperation and relationship between prescription drug benefits cost. -

Introducing the IMED Biotech Unit What Science Can Do Introduction What Science Can Do
Introducing the IMED Biotech Unit What science can do Introduction What science can do At AstraZeneca, our purpose is to push the Our IMED Biotech Unit applies its research and Our approach to R&D development capabilities and technologies to The IMED Biotech Unit plays a critical boundaries of science to deliver life-changing accelerate the progress of our pipeline. Through role in driving AstraZeneca’s success. Working together with MedImmune, medicines. We achieve this by placing science great collaboration across our three science units, our global biologics arm and Global we are confident that we can deliver the next wave Medicines Development (GMD), our at the centre of everything we do. late-stage development organisation, of innovative medicines to transform the lives of we are ensuring we deliver an innovative patients around the world. and sustainable pipeline. Pancreatic beta cells at different Eosinophil prior to Minute pieces of circulating tumour DNA stages of regeneration apoptosis (ctDNA) in the bloodstream IMED Biotech Unit MedImmune Global Medicines Development Focuses on driving scientific advances Focuses on biologics research and Focuses on late-stage development in small molecules, oligonucleotides and development in therapeutic proteins, of our innovative pipeline, transforming other emerging platforms to push the monoclonal antibodies and other next- exciting science into valued new boundaries of medical science. generation molecules to attack a range medicines and ensuring patients of diseases. around the world can access them. It’s science that compels us to push the boundaries of what is possible. We trust in the potential of ideas and pursue them, alone and with others, until we have transformed the treatment of disease. -

Esomeprazole: a New Proton Pump Inhibitor for NSAID-Associated Peptic Ulcers and Dyspepsia
DRUG PROFILE Esomeprazole: a new proton pump inhibitor for NSAID-associated peptic ulcers and dyspepsia Grace Lai-Hung Wong Ulcer and ulcer symptoms related to the use of non-steroidal anti-inflammatory drugs & Joseph JY Sung† (NSAIDs) and aspirin constitute a major global health issue. Despite various attempts to †Author for correspondence prevent and heal injuries inflicted by NSAIDs and aspirin, acid suppression remains one of The Prince of Wales Hospital, Department of Medicine and the cornerstones in the management of NSAID-associated ulcers. Esomeprazole, the Therapeutics, 9/F, S-optical isomer (enantiomer) of omeprazole, suppresses gastric acid secretion by 30–32 Ngan Shing Road, inhibiting the parietal cell membrane enzyme H+/K+-ATPase. With improved bioavailability, Shatin, NT, Hong Kong SAR due to reduced first-pass metabolism in the liver, esomeprazole promises to be more Tel.: +852 2632 3132 potent in acid suppression in the stomach. Similar to omeprazole, the safety profile of Fax: +852 2645 1699 esomeprazole has been well established. Clinical studies comparing esomeprazole with [email protected] other proton pump inhibitors (PPIs) in the healing of NSAID-related ulcer are few. Recent multicenter randomized studies demonstrated that esomeprazole significantly improves dyspeptic symptoms in patients taking nonselective NSAIDs and specific cyclooxygenase-2 inhibitors. Esomeprazole also protects the stomach from aspirin-induced ulcer bleeding. Safety profiles of esomeprazole appear promising. Non-steroidal anti-inflammatory drugs both basal and stimulated acid secretion. PPIs are (NSAIDs) are the most commonly used medica- most effective against meal-induced gastric acid tions for chronic pain and arthritis due to their secretion [3]. -

Dr. Reddy's – Recall of Ranitidine
Dr. Reddy’s – Recall of ranitidine • On October 23, 2019, Dr. Reddy’s announced the voluntary, consumer-level recall of prescription ranitidine due to potential contamination with N-nitrosodimethylamine (NDMA). • Dr. Reddy’s initially announced retail level recalls for prescription and OTC ranitidine in early October; however, the recall of prescription ranitidine products has been escalated to the consumer level. The recall of OTC ranitidine remains at the retail level. Prescription Ranitidine Capsules Recalled by Dr. Reddy’s Product Description NDC# Ranitidine 150 mg capsules, 60 count bottle 55111-129-60 Ranitidine 150 mg capsules, 500 count bottle 55111-129-05 Ranitidine 300 mg capsules, 30 count bottle 55111-130-30 Ranitidine 300 mg capsules, 100 count bottle 55111-130-01 • Refer to the Dr. Reddy’s announcement for a complete list of the OTC recalled ranitidine products. • Other manufacturers, including Apotex, Perrigo and Sanofi have recently announced retail level recalls of their OTC ranitidine products. • In September, Sandoz issued a consumer level recall of prescription ranitidine products. • These recalls follow a recent FDA statement about NDMA impurities detected in ranitidine medicines. • NDMA is classified as a probable human carcinogen based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables. • Ranitidine is an OTC and prescription drug. Ranitidine is an H2 (histamine-2) blocker, which decreases the amount of acid created by the stomach. OTC ranitidine is approved to prevent and relieve heartburn associated with acid ingestion and sour stomach. • Prescription ranitidine is approved for multiple indications, including treatment and prevention of ulcers of the stomach and intestines and treatment of gastroesophageal reflux disease. -

BOOKTIVISM: the Power of Words
BOOKTIVISM: The Power of Words Book•ti•vi•sm(noun). 1. The mobilization of groups of concerned citizens produced by reading books offering powerful analyses of social or political issues. 2. A call to action based on the sharing of knowledge through books. 3. Books + activism = “booktivism.” 4. A term first used at the SellingSickness, 2013: People Before Profits conference in Washington, DC, see www.sellingsickness.com. Read. Discuss. Be thoughtful. Be committed. Here are some more suggestions to get you started: 1) Set up a reading group on disease-mongering among interested friends and colleagues. If you do The books included in BOOKTIVISM celebrate recent contributions to the broad topic of disease- not already have a group of interested readers, post a notice in your workplace, library, community mongering, especially as they examine the growing prevalence and consequences of overtreatment, center, apartment building, etc. Once you have a group, decide where to meet. Book clubs can overscreening, overmarketing, and overdiagnosis (see Lynn Payer’s 1992 classic, Disease-Mongers: How meet anywhere – at homes, in dorms, in pubs, in coffeehouses, at libraries, even online! Decide on Doctors, Drug Companies, and Insurers Are Making You Feel Sick, for an introduction to timing and format. Will you meet monthly/bimonthly? You’ll need time to prepare for the sessions, disease-mongering). but not so much time that you lose touch. Circulate the reading guide. It is usually best if one person leads each discussion, to have some questions at the ready and get things rolling. Although the challenge to disease-mongering is not unprecedented (the women’s health movement of the 1970s was another key historical moment), these books represent an impressive groundswell OR, maybe you’d like to of amazing, powerful, brilliant, and often deeply unsettling investigations by physicians, health scientists, 2) Set up a lecture/discussion group. -

Label Phase of the Study
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ------------------------WARNINGS AND PRECAUTIONS---------------- NEXIUM safely and effectively. See full prescribing information for • Symptomatic response does not preclude the presence of gastric NEXIUM. malignancy (5.1) • Atrophic gastritis has been noted with long-term omeprazole therapy NEXIUM (esomeprazole magnesium) Delayed-Release Capsules, for Oral (5.2) Use • PPI therapy may be associated with increased risk of Clostridium NEXIUM (esomeprazole magnesium) For Delayed-Release Oral difficile associated diarrhea. (5.3) Suspension • Bone Fracture: Long-term and multiple daily dose PPI therapy may be Initial U.S. Approval: 1989 (omeprazole) associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5.4) -----------------------RECENT MAJOR CHANGES----------------------- • Hypomagnesemia has been reported rarely with prolonged treatment Warnings and Precautions, Clostridium difficile associated 09/2012 with PPIs (5.5) diarrhea (5.3) • Avoid concomitant use of NEXIUM with St John’s Wort or rifampin Warnings and Precautions, Concomitant use of NEXIUM with due to the potential reduction in esomeprazole levels (5.6) (7.3) Methotrexate (5.10) 01/2012 • Interactions with diagnostic investigations for Neuroendocrine Tumors: Increases in intragastric pH may result in hypergastrinemia and -----------------------INDICATIONS AND USAGE----------------------- enterochromaffin-like cell hyperplasia and -

Proton Pump Inhibitors Aciphex
PHARMACY PRE-AUTHORIZATION CRITERIA DRUG (S) Proton Pump Inhibitors Aciphex (Brand name) Dexilant Nexium/esomeprazole (RX Version) Omeprazole-bicarbonate Prevacid (Brand name) Prilosec (Brand name) Protonix (Brand name) Zegerid (RX Version and Brand name) POLICY # 14157 INDICATIONS These agents are approved for the treatment of duodenal ulcers, gastroesophageal reflux disease (GERD), Zollinger Ellison syndrome and H. pylori eradication. CRITERIA DRUG FREEDOM DRUG LIST Step COMMERCIAL DRUG LIST Step Requirements Requirements Aciphex (brand All of these: All of these: name) • Nexium OTC • Nexium OTC • Prilosec OTC , omeprazole, • Prilosec OTC , omeprazole, or Zegerid OTC or Zegerid OTC • Prevacid 24HR OTC/ • Prevacid 24HR OTC/ lansoprazole lansoprazole • Protonix/pantoprazole • Protonix/pantoprazole Dexilant All of these: ALL of these: • Nexium OTC • Nexium OTC • Prilosec OTC , omeprazole, • Prilosec OTC , omeprazole, or Zegerid OTC or Zegerid OTC • Prevacid 24HR OTC/ • Prevacid 24HR OTC/ lansoprazole lansoprazole • Protonix/pantoprazole • Protonix/pantoprazole esomeprazole All of these: All of these: • Nexium OTC • Nexium OTC • Prilosec OTC , omeprazole, • Prilosec OTC , omeprazole, or Zegerid OTC or Zegerid OTC • Prevacid 24HR OTC/ • Prevacid 24HR OTC/ PHARMACY PRE-AUTHORIZATION CRITERIA lansoprazole lansoprazole • Protonix/pantoprazole • Protonix/pantoprazole lansoprazole PA Not Required PA Not Required Nexium (RX All of these: All of these: Version) • Nexium OTC • Nexium OTC • Prilosec OTC , omeprazole, • Prilosec OTC , omeprazole, or -

Astrazeneca 17Th January 2013 Attractive Risk-Reward As New CEO Comes in Healthcare Fair Value 3440P Vs
INDEPENDENT RESEARCH AstraZeneca 17th January 2013 Attractive risk-reward as new CEO comes in Healthcare Fair Value 3440p vs. 2860p (price 3,042p) BUY vs. NEUTRAL Bloomberg AZN LN Among the big names in the pharmaceutical industry, AstraZeneca is Reuters AZN.L obviously one of the few which carries in principle one of the most 12-month High / Low (p) 3,112 / 2,591 significant upsides considering its current valuation, provided the new Market capitalisation (GBPm) 37,921 Enterprise Value (BG estimates GBPm) 39,062 CEO, Pascal Soriot, is able not only to present a comprehensive and Avg. 6m daily volume ('000 shares) 2,247 clear strategy but also to package his speech in an attractive manner Free Float 100% for the investment community to jump in as early as the beginning of 3y EPS CAGR [2012-2015] -1.2% Gearing (12/11) 8% 2013. Dividend yields (12/12e) 5.72% On 31 January 2013, Pascal Soriot is expected to present his initial YE December 12/11 12/12e 12/13e 12/14e thoughts about how to drive AstraZeneca forward and which strategy to Revenue (USDm) 33,591 28,081 27,524 27,251 EBIT(USDm) 12,795 7,921 7,818 8,334 implement. Since he took over as CEO on 1 October 2012, he has spent Basic EPS (USD) 7.33 4.81 4.58 5.04 much time meeting people within the group and also key shareholders to Core EPS (USD) 7.72 6.71 6.13 6.33 EV/Sales 1.9x 2.2x 2.2x 2.0x make the best possible assessment of the situation and to hear their EV/EBITDA 4.1x 6.0x 5.6x 5.0x expectations and hopes before presenting a roadmap. -

Pharmaceuticals in the Environment
Pharmaceuticals in the Environment As a responsible healthcare company, we are committed to the health and safety of our society and planet. We make it a priority to effectively manage the risks associated with pharmaceuticals in the environment (PIE). 1 How do Pharmaceutical Products get into the Environment? Pharmaceuticals enter the environmental mainly as AstraZeneca supports actions based on scientific a result of patient use, where they can pass through evidence to address the challenges presented by PIE. our bodies and into waterways. Drug manufacture and These include: the improper disposal of unused medicines also add • Assessing the environmental risk of our medicines to the trace levels of pharmaceuticals in rivers, lakes, to support drug marketing authorisations soils, and, sometimes, drinking water. AstraZeneca recognises that, even in such low concentrations, • Actively managing the environmental risks resulting the risks associated with Pharmaceuticals in the from our manufacturing Environment (PIE) should be determined, minimised • Supporting industry and government efforts to and managed. improve medicine disposal programs and education • Co-sponsoring research to fill scientific gaps 88% to understand and mitigate the risks of PIE. as a result of patient use To learn more about the risks associated with PIE and what we are doing to manage them please click here. 2% attributed to waste Pharmaceuticals from production in the environment 10% from unused medicines that people don’t dispose of properly 2 Societal Concerns about PIE Trace amounts of pharmaceuticals have been detected in the environment for more than 20 years. As environmental monitoring expands and the methods for measuring pharmaceuticals in the environment become more sophisticated and detection limits get better, the geographical scope and number of pharmaceuticals measured in the environment will grow.