Introducing the IMED Biotech Unit What Science Can Do Introduction What Science Can Do
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detection of Dna Hybridization on a Configurable Digital Microfluidic
DETECTION OF DNA HYBRIDIZATION ON A CONFIGURABLE DIGITAL MICROFLUIDIC BIO- CHIP USING SPR IMAGING Lidija Malic1, Teodor Veres2 and Maryam Tabrizian1 1Biomedical Engineering Department, McGill University, CANADA and 2Industrial Materials Institute, National Research Council, CANADA ABSTRACT This paper presents a configurable digital microfluidic-based surface plasmon resonance (SPR) biochip platform comprising an electrowetting-on-dielectric (EWOD) microfluidic device coupled to SPR imaging (SPRi). We demonstrate its application for dynamic on-chip simultaneous immobilization of different DNA probes in combination with multichannel label-free real-time detection of subse- quent hybridization reactions. The integrated EWOD-SPRi system would enable the development of high-throughput, rapid and ultrasensitive biomolecular detection strategies beyond DNA microarray applications. KEYWORDS: Digital microfluidics, EWOD, SPR imaging, DNA hybridization INTRODUCTION EWOD microfluidics has attracted considerable attention in the past decade and the most recent efforts are directed towards its application in biomedical research [1- 3]. While these studies demonstrate the versatility of EWOD devices, the reported applications involve homogeneous phase reactions [4] and detection methods that require labeled biomolecules [2] or sample extraction from the chip [1]. This in- creases both the time and complexity of the assay. To introduce new applications relying on label-free, real-time surface sensitive detection techniques such as SPRi, it would be advantageous to use droplet-based EWOD actuation for surface specific biomolecule immobilization. However, the need of hydrophobic properties for EWOD actuation renders immobilization of biomolecules such as DNA on the sur- face of the chip impossible [3, 4]. In this paper, we demonstrate for the first time the use of an EWOD microfluidic chip to dynamically immobilize DNA probes in a two-dimensional array, followed by SPRi detection of bioaffinity interactions. -

Structure and Function Of
8/5/2019 Micro Array /DNA chip` It is collection of microscopic DNA spots attached to a solid surface usually glass, plastic or silicon biochip Each DNA spot contains • picomoles (10−12 moles) of a specific DNA sequence • known as probes or reporters . • These can be a short section of a gene • Other DNA element that are used to hybridize a cDNA The original nucleic acid arrays were macro arrays approximately 9 cm × 12 cm Micro Array /DNA chip` Micro Array /DNA chip` cRNA sample (called target) • Probe-target hybridization • Detected • Quantified • detection of fluorophore- silver-, or chemiluminescence-labeled targets Example of an approximately 40,000 probe spotted 1 8/5/2019 Micro Array /DNA chip` Micro Array/ DNA chip` Use • To determine relative abundance of nucleic acid sequences in the target. • To measure the expression levels of large numbers of genes simultaneously • detect RNA (most commonly as cDNA after reverse transcription) • To genotype multiple regions of a genome. Microarray Analysis Techniques Microarray Analysis Techniques Microarray manufacturers LIMMA • Affymetrix • A set of tools for background correction • Agilent MA plots Comparing two different arrays • To plot the data. • Two different samples • R, MATLAB, and Excel • Hybridized to the same array • For adjustments for systematic errors introduced • Differences in procedures • Dye intensity effects. 2 8/5/2019 Protein synthesis • Messenger RNA (mRNA) molecules direct Median polish Algorithm the assembly of proteins on ribosomes. The median polish is an exploratory data analy sis procedure proposed by the statistician John Tukey. • Transfer RNA (tRNA) molecules are used Data Normalization to to deliver amino acids to the ribosome • Ribosomal RNA (rRNA) then links amino acids together to form proteins. -

Lab-On-Chip Microdevices for Capturing, Imaging and Counting White Blood Cells
LAB-ON-CHIP MICRODEVICES FOR CAPTURING, IMAGING AND COUNTING WHITE BLOOD CELLS by Anurag Tripathi A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Mechanical Engineering) in The University of Michigan 2012 Doctoral Committee: Associate Professor Nikolaos Chronis, Chair Associate Professor Katsuo Kurabayashi Assistant Professor Jianping Fu Assistant Professor Sunitha Nagrath To The Almighty And To My Family & Friends ii ACKNOWLEDGEMENTS It is my honor and absolute pleasure to acknowledge people who made this thesis possible. First and foremost, I would like to thank my advisor Dr. Nikos Chronis whose guidance and training enabled me to develop skills in microfabrication and microfluidics technology. More than the technical knowledge I gained from Dr. Chronis in the past five years, he taught me the virtues of hard work, sincerity and integrity. In the toughest of situations, his patience and understanding provided me with courage and confidence and enabled me to see through those hard times. He inculcated a strong aptitude for research in me and his ‘never give up’ principle left an indelible impression on my problem solving approach. I would be eternally grateful to Dr. Chronis for mentoring me and transforming me into a better researcher and a better human being. A big gratitude is in order for the other three members of my thesis dissertation committee, Dr. Katsuo Kurabayashi, Dr. Jianping Fu and Dr. Sunitha Nagrath. It was a privilege to have them on my committee. I thank them wholeheartedly for their valuable inputs during the Ph.D. preliminary examinations. I acknowledge the encouragement they provided to my research and would like to thank them for their support and motivation all this while. -

The Bio Revolution: Innovations Transforming and Our Societies, Economies, Lives
The Bio Revolution: Innovations transforming economies, societies, and our lives economies, societies, our and transforming Innovations Revolution: Bio The The Bio Revolution Innovations transforming economies, societies, and our lives May 2020 McKinsey Global Institute Since its founding in 1990, the McKinsey Global Institute (MGI) has sought to develop a deeper understanding of the evolving global economy. As the business and economics research arm of McKinsey & Company, MGI aims to help leaders in the commercial, public, and social sectors understand trends and forces shaping the global economy. MGI research combines the disciplines of economics and management, employing the analytical tools of economics with the insights of business leaders. Our “micro-to-macro” methodology examines microeconomic industry trends to better understand the broad macroeconomic forces affecting business strategy and public policy. MGI’s in-depth reports have covered more than 20 countries and 30 industries. Current research focuses on six themes: productivity and growth, natural resources, labor markets, the evolution of global financial markets, the economic impact of technology and innovation, and urbanization. Recent reports have assessed the digital economy, the impact of AI and automation on employment, physical climate risk, income inequal ity, the productivity puzzle, the economic benefits of tackling gender inequality, a new era of global competition, Chinese innovation, and digital and financial globalization. MGI is led by three McKinsey & Company senior partners: co-chairs James Manyika and Sven Smit, and director Jonathan Woetzel. Michael Chui, Susan Lund, Anu Madgavkar, Jan Mischke, Sree Ramaswamy, Jaana Remes, Jeongmin Seong, and Tilman Tacke are MGI partners, and Mekala Krishnan is an MGI senior fellow. -

Guide to Biotechnology 2008
guide to biotechnology 2008 research & development health bioethics innovate industrial & environmental food & agriculture biodefense Biotechnology Industry Organization 1201 Maryland Avenue, SW imagine Suite 900 Washington, DC 20024 intellectual property 202.962.9200 (phone) 202.488.6301 (fax) bio.org inform bio.org The Guide to Biotechnology is compiled by the Biotechnology Industry Organization (BIO) Editors Roxanna Guilford-Blake Debbie Strickland Contributors BIO Staff table of Contents Biotechnology: A Collection of Technologies 1 Regenerative Medicine ................................................. 36 What Is Biotechnology? .................................................. 1 Vaccines ....................................................................... 37 Cells and Biological Molecules ........................................ 1 Plant-Made Pharmaceuticals ........................................ 37 Therapeutic Development Overview .............................. 38 Biotechnology Industry Facts 2 Market Capitalization, 1994–2006 .................................. 3 Agricultural Production Applications 41 U.S. Biotech Industry Statistics: 1995–2006 ................... 3 Crop Biotechnology ...................................................... 41 U.S. Public Companies by Region, 2006 ........................ 4 Forest Biotechnology .................................................... 44 Total Financing, 1998–2007 (in billions of U.S. dollars) .... 4 Animal Biotechnology ................................................... 45 Biotech -

Lab on a Chip Systems for Biochemical Analysis, Biology and Synthesis
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Lab on a Chip Systems for Biochemical Analysis, Biology and Synthesis Towards Simple, Scalable Microfabrication Technologies Based on COC and LTCC Miguel Berenguel Alonso Tesi Doctoral Programa de Doctorat en Qu´ımica Directors: Mar Puyol and Juli´anAlonso Chamarro Departament de Qu´ımica Facultat de Ci`encies 2017 Mem`oriapresentada per aspirar al Grau de Doctor per Miguel Berenguel Alonso Vist i plau Mar Puyol Juli´anAlonso Chamarro Professora Agregada Catedr`atic Departament de Qu´ımica Departament de Qu´ımica Bellaterra, 6 de Juny de 2017 iii The present dissertation was carried out with the following financial support: CTQ2009-12128 Nuevas plataformas microflu´ıdicaspara la miniaturizaci´on de sistemas (bio)anal´ıticos integrados e intensificaci´onde procesos de producci´onde nanomateriales. Ministerio de Ciencia e Innovaci´on,co- funded by FEDER. 2009SGR0323 Convocat`oriade suport als Grups de Recerca de Catalunya. Departament d'Universitats, Recerca i Societat de la Informaci´o,Gen- eralitat de Catalunya. FI-DGR 2012 Pre-doctoral scholarship FI-DGR granted by the Ag`enciade Gesti´od'Ajuts Universitaris i de Recerca, Generalitat de Catalunya, and co-funded by the ESF. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

Pharmaceuticals in the Environment
Pharmaceuticals in the Environment As a responsible healthcare company, we are committed to the health and safety of our society and planet. We make it a priority to effectively manage the risks associated with pharmaceuticals in the environment (PIE). 1 How do Pharmaceutical Products get into the Environment? Pharmaceuticals enter the environmental mainly as AstraZeneca supports actions based on scientific a result of patient use, where they can pass through evidence to address the challenges presented by PIE. our bodies and into waterways. Drug manufacture and These include: the improper disposal of unused medicines also add • Assessing the environmental risk of our medicines to the trace levels of pharmaceuticals in rivers, lakes, to support drug marketing authorisations soils, and, sometimes, drinking water. AstraZeneca recognises that, even in such low concentrations, • Actively managing the environmental risks resulting the risks associated with Pharmaceuticals in the from our manufacturing Environment (PIE) should be determined, minimised • Supporting industry and government efforts to and managed. improve medicine disposal programs and education • Co-sponsoring research to fill scientific gaps 88% to understand and mitigate the risks of PIE. as a result of patient use To learn more about the risks associated with PIE and what we are doing to manage them please click here. 2% attributed to waste Pharmaceuticals from production in the environment 10% from unused medicines that people don’t dispose of properly 2 Societal Concerns about PIE Trace amounts of pharmaceuticals have been detected in the environment for more than 20 years. As environmental monitoring expands and the methods for measuring pharmaceuticals in the environment become more sophisticated and detection limits get better, the geographical scope and number of pharmaceuticals measured in the environment will grow. -

Comparison of Specificity and Sensitivity of Biochip and Real-Time Polymerase Chain Reaction for Pork in Food
Journal of Food and Nutrition Research (ISSN 1336-8672) Vol. 58, 2019, No. 3, pp. 225–235 Comparison of specificity and sensitivity of biochip and real-time polymerase chain reaction for pork in food ZuZana DrDolová – Ľubomír belej – joZef Golian – lucia benešová – joZef Čurlej – Peter Zajác Summary Adulteration and food fraud are serious global problems. Analytical methods regarding animal species should repre- sent a comprehensive tool for identification and possible quantification of the components. The aim of the study was to confirm usability and sensitivity of the MEAT 5.0 LCD-Array Kit (version 5.0, Chipron, Berlin, Germany) for forensic analysis of food, which simultaneously detects 24 kinds of animals. We verified its reliability for identification of pork mixed with meat of other 15 animal species. Mixtures of animal species meat were tested in the raw state and after heat treatment. For comparison, real-time polymerase chain reaction innuDETECT Pork Assay (Analytic Jena, Berlin, Germany) with quantitative potential was used. A standard pork DNA curve was established and the detection limit was determined. MEAT 5.0 LCD-Array Kit was found to have a high degree of robustness, probe specificity and repeat- ability, with a detection limit down to 0.5 % (w/w) but did not allow quantification. We also used both test methods for analysis of commercial products. By these analyses, we identified the presence of pork DNA in 14 products for which this fact was not reported on the product label. Keywords meat; pork; biochip; detection; real-time polymerase chain reaction Following European Union labelling regula- the horse meat scandal uncovered in Europe in tions [1], meat products should be accurately la- 2013, when undeclared horse meat was substi- belled regarding their species content. -

Monoclonals 5/10/06 11:51 Page 17
Monoclonals 5/10/06 11:51 Page 17 Therapeutics MONOCLONALS the billion dollar molecules of the future In 1975, two British scientists thought of creating a mouse antibody that could be replicated or ‘cloned’ to produce identical copies. Identical copies with identical modes of action had the potential to be a drug.They had launched what was to be one of biotechnology’s best ideas. If the pair could mimic the immune system’s ‘seek and destroy’ capability by pre-designing antibodies for all manner of disease targets, it should be possible to block or activate cellular activity to order. It was an elegant concept opening up a raft of therapeutic possibilities, particularly in the area of cancer. Here it might be possible to attach a chemotherapy drug to an antibody.The antibody’s specificity for a particular disease target would guide the chemo drug only to the cancerous cells and not the healthy ones.The concept, therefore, was excellent, but the use of mice monoclonals had drawbacks.The human body did not like antibodies from mice anymore than it liked the flu virus, so the antibodies themselves triggered an immune response and were destroyed.This ‘immunogenicity’ issue was to occupy researchers for another 20 or so years before it became possible to craft a monoclonal antibody (mAb) that would be acceptable to the human immune system. fter rather a long period in the desert, untreatable diseases. A further six lead drugs are in By Dr Martin Wiles monoclonal antibody drugs are once the final stages of clinical trials and approximately and Patrik A again generating a wave of excitement. -
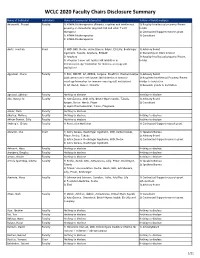
WCLC 2020 Faculty Chairs Disclosure Summary
WCLC 2020 Faculty Chairs Disclosure Summary Name of Individual Individual's Name of Commercial Interest(s) Nature of Relationship(s) Adusumilli, Prasad FacultyRole(s) in Activity 1) ATARA Biotherapeutics (Patents, royalties and intellectual 1) Royalty/Intellectual property/Patent property on mesothelin-targeted CAR and other T-cell holder therapies) 2) Contracted/Support research grant 2) ATARA Biotherapeutics 3) Consultant 3) ATARA Biotherapeutics Aerts, Joachim Chair 1) MSD, BMS, Roche, Astra-Zeneca, BAyer, Eli-Lilly, Boehringer 1) Advisory Board Ingelheim, Takeda, Amphera, BIOCAD 2) Ownership or Stock interest 2) Amphera 3) Royalty/Intellectual property/Patent 3) allogenic tumor cell lysate/JAK inhibition in holder immunooncology/ biomarker for immuno-oncology (all institution) Aggarwal, Charu Faculty 1) BMS, ROCHE, AZ, MERCK, Celgene, BluePrint, Diachaii Sankyo 1) Advisory Board 2)Allogenic tumor cell lysate/JAK inhibition in immuno- 2) Royalties/Intellectual Property/Patent oncology/biomarker for immuno-oncology (all institution) Holder to institution 3) AZ, Merck, Xencor, Novartis 3) Research grants to institution Agrawal, Abhinav Faculty Nothing to disclose Nothing to disclose Ahn, Myung-Ju Faculty 1) AstraZeneca, MSD, Lilly, Bristol-Myers Squibb, Takeda, 1) Advisory Board Amgen, Roche, Merck, Pfizer 2) Consultant 2) Alpha Pharmaceutical, Yuhan, Progenere Aisner, Dara Faculty Nothing to disclose Akerley, Wallace Faculty Nothing to disclose Nothing to disclose Akhtar-Danesh, Gilly Faculty Nothing to disclose Nothing to disclose -

Development of an Acousto-Electric Biochemical Sensor (AEBS) for Monitoring Biological and Chemical Processes
Development of an Acousto-Electric Biochemical Sensor (AEBS) For Monitoring Biological and Chemical Processes A Thesis Submitted to the Faculty of Drexel University by Michel M. A. Francois in partial fulfillment of the requirements for the degree of Doctor of Philosophy June 2008 © Copyright 2008 Michel M. A. François. All Rights Reserved. iii Dedications To my daughters, Michele-Olivia François and Coralie-Michele François. iv Table of Contents LIST OF TABLES ...............................................................................................................x LIST OF ILLUSTRATIONS ............................................................................................ xii ABSTRACT ................................................................................................................... xxvi 1. CHAPTER 1: Introduction – Importance of sensors in our life ...............................1 1.1 Objective ....................................................................................................................2 1.2 Example of a Basic Complex Sensor System ............................................................2 1.3 Present Methods for Identifying and Sensing ............................................................5 1.4 Need for Cost Effective, Fast and Accurate Small Portable System .........................6 1.5 Areas most in Need of Miniature Bio-Sensors ...........................................................7 1.5.1 Medical ..............................................................................................................7