1999 Annual Report and Form 20-F CONTENTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chief Executive Officer Graham Baker
OVERVIEW OUR BUSINESS OPERATIONAL FINANCIAL RISK GOVERNANCE ACCOUNTS 48 & MARKETPLACE REVIEW REVIEW OUR BOARD OF DIRECTORS ROBERTO QUARTA (67) OLIVIER BOHUON (58) GRAHAM BAKER (48) CHAIRMAN CHIEF EXECUTIVE OFFICER CHIEF FINANCIAL OFFICER Joined the Board in December 2013 and Joined the Board and was appointed Chief -RLQLQJWKH%RDUGDV&KLHI)LQDQFLDO2I²FHULQ appointed Chairman following election by ([HFXWLYH2I²FHULQ$SULO+HUHVLJQHGDV March 2017. shareholders at the April 2014 Annual General a Member of the Nomination & Governance Meeting. He was also appointed Chairman of &RPPLWWHHRQ)HEUXDU\ CAREER AND EXPERIENCE the Nomination & Governance Committee and Graham holds an MA degree in Economics from a Member of the Remuneration Committee on CAREER AND EXPERIENCE Cambridge University and qualified as a Chartered that day. Olivier holds a doctorate in Pharmacy from the Accountant and Chartered Tax Advisor with Arthur University of Paris and an MBA from HEC, Paris. Andersen. In 1995, he joined AstraZeneca PLC where CAREER AND EXPERIENCE He started his career in Morocco with Roussel Uclaf he worked for 20 years, holding multiple senior roles, Roberto is a graduate and a former Trustee of the S.A. and then, with the same company, held a number including Vice President, Finance, International (2013- College of the Holy Cross, Worcester (MA), US. of positions in the Middle East with increasing levels of 2015) with responsibility for all emerging markets, He started his career as a manager trainee at David responsibility. He joined Abbott in Chicago as head of Vice President, Global Financial Services (2011-2013) Gessner Ltd, before moving on to Worcester Controls their anti-infective franchise with Abbott International and Vice President Finance & Chief Financial Officer, Corporation and then BTR plc, where he was a before becoming Pharmaceutical General Manager North America (2008-10). -
Analyst Report
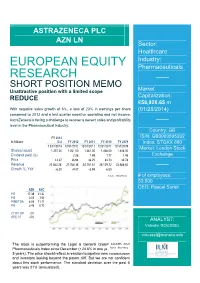
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Affymetrix and the "DNA Chip" Revolution
taken pride in placing in the top 10 among the The Evolution of Astra best companies to work for in a number of ∗ magazine surveys, from Fortune to Working Merck Inc. in 1998 Mother. Merck & Co. sales grew from $9.6 billion in 1992 to $23 billion by the end of "We plan to revolutionize the pharmaceutical industry by 19972, topping the world drug sales league in being the best at linking patients and products.” Wayne Yetter, President of Astra Merck Inc. both years. In fact, Merck has seen its global market share jump one-third in the 1992-1998 period to edge Glaxo Wellcome in 1997 with a Section I. Background 3 4.6% world market share. In contrast to the In 1982, Merck & Co. and Astra AB signed an strategy of other pharmaceutical companies such agreement by which products resulting from as Glaxo, this growth was largely fueled by Astra’s research pipeline would be taken through internal R&D efforts as opposed to mergers and clinical trials and registration, to be ultimately acquisitions. marketed and sold by Merck in the United States. The initial terms of the contract specified Another major strategic move made to enhance that Astra’s products would be transferred to its competitive position was the acquisition of Merck’s development and marketing Medco Containment Services Inc. in 1993 organizations, with Astra receiving royalties on (renamed Merck-Medco Managed Care). the sales of the Astra drugs.1 Merck-Medco provides pharmaceutical benefit services in the United States to control The initial cooperation and relationship between prescription drug benefits cost. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

How to Sustain Healthcare Transformations
Voices on transformation A marathon, not a sprint: How to sustain healthcare transformations Voices on transformation is written by experts and practitioners in McKinsey & Company’s Pharmaceuticals and Medical Products Practice. To send comments or request copies of this publication, please email us at [email protected] Editors: Gayane Gyurjyan, Ioana Parsons, Shail Thaker, Jill Willder, Carla Zwaanstra Artwork and design: Afitha de Rijk-Voeten Portrait illustrations: Allan Burch Special thanks to: Lucia Darino, Martin Dewhurst, Claudio Feser, Vincent Forlenza, Jane Griffiths, Judith Hazlewood, Nadine Mansour, Angelika Reich, Pascal Soriot, David Speiser, Kirsten Westhues, André Wyss Copyright © 2016 McKinsey & Company. All rights reserved. This publication is not intended to be used as the basis for trading in the shares of any company or for undertaking any other complex or significant financial transaction without consulting appropriate professional advisers. No part of this publication may be copied or redistributed in any form without the prior written consent of McKinsey & Company. 2 PMP Voices on transformation <Chaper title> Contents Introduction 4 Cracking the code: How successful pharma leaders 7 manage transformations A health check for pharma: Overcoming change fatigue 15 in the pharmaceutical industry Putting science at the heart of renewed purpose 26 An interview with Pascal Soriot, AstraZeneca Transforming a medical devices company into a solutions provider 34 An interview with Vincent Forlenza, Becton Dickinson Refocusing the business around patient outcomes 42 An interview with Jane Griffiths, Janssen EMEA NBS: Creating value across Novartis 48 An interview with André Wyss, Novartis Voices on digital: How pharma can win in a digital world 57 About the authors 66 3 Introduction Over the past decade, the pharmaceutical industry has been struggling to keep up with rapid and dramatic changes in the external environment. -

Astrazeneca Annual Report 2006
AND FORM 20-F ANNUAL INFORMATION REPORT 2006 CONTENTS 2006 IN BRIEF 1 FINANCIAL STATEMENTS 19. Reserves 120 CHAIRMAN’S STATEMENT 2 Preparation of the Financial Statements 20. Minority interests 121 CHIEF EXECUTIVE OFFICER’S REVIEW 3 and Directors’ Responsibilities 96 21. Dividends to shareholders 121 FINANCIAL HIGHLIGHTS 6 Directors’ Responsibilities for, and Report 22. Acquisitions of on, Internal Control over Financial DIRECTORS’ REPORT business operations 121 Reporting 96 Business review 8 23. Disposal of business operations 123 Auditors’ Reports on the Financial 24. Post-retirement benefi ts 123 > Business environment 9 Statements and on Internal Control over > Strategy 11 Financial Reporting (Sarbanes-Oxley Act 25. Employee costs and share option plans for employees 128 > Our resources, skills Section 404) 97 and capabilities 12 Independent Auditors’ Report to the 26. Commitments and contingent liabilities 133 > Measuring performance 15 Members of AstraZeneca PLC (Group) 97 27. Leases 146 > Therapy area review Consolidated Income Statement 98 28. Statutory and other information 146 – Cardiovascular medicines 16 Consolidated Statement of Recognised Income and Expense 98 29. Share capital of parent – Gastrointestinal medicines 20 Consolidated Balance Sheet 99 company 147 – Neuroscience medicines 23 Consolidated Cash Flow Statement 100 Principal Subsidiaries 148 – Oncology medicines 26 Accounting Policies (Group) 101 Additional Information for US Investors 149 – Respiratory and Independent Auditors’ Infl ammation medicines 29 Notes to the Financial Statements (Group) Report to the Members of – Infection medicines 32 AstraZeneca PLC (Company) 157 1. Operating profi t 104 > Geographic review 33 Company Balance Sheet 158 2. Profi t on sale of interest > Research and development 37 in joint venture 104 Accounting Policies (Company) 159 > Development pipeline table 40 3. -

Zusammenfassung Dit-Votum Vom 01.01.2005 Bis 31.12.2005
Zusammenfassung dit-Votum vom 01.01.2005 bis 31.12.2005 ISIN Gesellschaft Antrag- Mgmt- dit- Datum-HV Tagesordnungspunkt steller Empfehlung Votum Art-HV ES0105200416 Abengoa S.A. 25.06.2005 AGM 1 Genehmigung von Einzel- und Konzernjahresabschluss für das Geschäftsjahr 2004; Mgmt für für Genehmigung der Entlastung der Directors 2 Genehmigung der Gewinnzuweisung Mgmt für für 3 Wahl der Abschlussprüfer für Unternehmen und konsolidierten Konzern Mgmt für für 4 Änderung von Artikel 39 bezüglich: Erhöhung der maximalen Anzahl an Mgmt für für Vorstandsmitgliedern von 7 auf 9 5 Wiederwahl, Nominierung und/oder Bestätigung von Mitgliedern des Vorstands Mgmt für für 6 Ermächtigung zur Ausgabe von Aktien oder aktiengebundenen Anleihen ohne Mgmt für gegen Bezugsrechte 7 Ermächtigung zur zusätzlichen Ausgabe von wandelbaren und nicht wandelbaren Mgmt für für Schuldverschreibungen und/oder Schuldpapieren 8 Genehmigung zum Rückkauf von Anteilen Mgmt für für 9 Genehmigung für Aufsichtsrat zur Ratifizierung und Umsetzung der genehmigten Mgmt für für Beschlüsse 10 Bestätigung des Versammlungsprotokolls Mgmt für für ES0105200416 Abengoa S.A. 15.10.2005 EGM 1 Vorlage des Aktienrückkaufplans; Ermächtigung des Aufsichtsrats zur Festlegung der Management Für Für Bedingungen für den Aktienrückkaufplan 2 Genehmigung zum Rückkauf von Anteilen Management Für Für 3 Genehmigung für den Aufsichtsrat zur Ratifizierung und Umsetzung der genehmigten Management Für Für Beschlüsse bezüglich: Aktienrückkauf 4 Bestätigung des Versammlungsprotokolls Management Für Für US00339B1070 Abgenix, Inc. 13.06.2005 AGM 1.1 Wahl von R. Scott Greer zum Director Mgmt für für 1.2 Wahl von M.K. Behrens, Ph.D., zum Director Mgmt für für 1.3 Wahl von R.S. Kucherlapati Ph.D., zum Director Mgmt für für 1.4 Wahl von Kenneth B. -

Astrazeneca: the First Abuse Case in the Pharmaceutical Sector Niklas FAGERLUND and Søren Bo RASMUSSEN, Directorate-General Competition, Unit B-2
Antitrust AstraZeneca: the first abuse case in the pharmaceutical sector Niklas FAGERLUND and Søren Bo RASMUSSEN, Directorate-General Competition, unit B-2 1. Introduction by AZ before patent offices in Belgium, Denmark, Germany, the Netherlands, Norway and the United On 15 June 2005 the Commission adopted a deci- Kingdom and before national courts in Germany sion (‘Decision’) fining the Swedish company and Norway. AstraZeneca AB and the UK company Astra- Zeneca Plc (together ‘AZ’) 60 million euros due to The misleading information was provided by AZ their infringements of Article 82 of the EC Treaty in the context of its two rounds of applications and Article 54 of the EEA Agreement. (in June 1993 and December 1994) to several pat- ent offices within the EEA for extra protection for The infringements involve misuses by AZ of public omeprazole (the active substance in AZ’s product procedures and regulations in a number of EEA Losec) in the form of so-called supplementary states aimed at excluding generic firms and paral- protection certificates (SPCs). Under the SPC Reg- lel traders from competing against AZ’s anti-ulcer ulation the basic patent protection for active sub- product Losec. stances in medicines can be extended by a maxi- In 1979, Astra AB (currently AstraZeneca AB), a mum of five years. Swedish research based company, had filed pat- The Decision raises no objections to AZ’s incorrect ent applications in Europe in respect of omepra- interpretation of the relevant legislation. Therefore, zole (the active substance in Losec). Losec’s basic the proceedings and outcome in Hässle AB v. -

Årsredovisning Med Information Från Form 20-F 2008 Inledning 2
ASTRAZENECA – ÅRSREDOVISNING MED INFORMATION FRÅN FORM 20-F 2008 INLEDNING 2 AstraZeneca och året i korthet 2 Utvecklingen i sammandrag 2 Ordföranden har ordet 4 Koncernchefens översikt 5 FÖRVALTNINGSBERÄTTELSE 8 FÖRVALTNINGSBERÄTTELSE 8 Inledning 8 Omvärldsanalys 9 Strategi, mål och mätetal 12 > Mäta våra resultat 14 > Vår utveckling – Ekonomiska och övriga nyckeltal 15 Resurser, kompetenser och möjligheter 16 > Läkemedel 16 > Forskning och utveckling 17 > Forsknings- och utvecklingsportfölj den 29 januari 2009 22 BOKSLUT 98 > Försäljning och marknadsföring 25 >6 Immateriella rättigheter 2 > Varuförsörjning och produktion 27 > Medarbetare 28 Ekonomisk översikt 31 > Rapporteringsprinciper 31 > Verksamhetsbakgrund och viktiga händelser 2008 32 > Kortfattad analys av perioden jan–dec 2008 33 > Finansiell ställning , inklusive kassafl öde och likviditet – 2008 35 > Omstrukturerings- och synergi- RAPPORT OM ERSÄTTNINGAR 174 kostnader 36 > Kapital och aktieavkastning 37 > Framtidsutsikter 37 > Kortfattad analys av perioden jan–dec 2007 38 > Finansiell ställning, inklusive kassafl öde och likviditet – 2007 40 > Hantering av fi nansiella risker 41 > Viktiga redovisningsprinciper och bedömningar 43 > Övrig redovisnings information 47 YTTERLIGARE INFORMATION 190 VIKTIG INFORMATION FÖR LÄSARE som var tillgänglig vid utarbetandet av denna Användandet av redovisad utveckling, fasta AV DENNA REDOVISNING årsredovisning med information från Form 20-F och valutakurser och ekonomiska mått för kärn- företaget åtar sig inget ansvar för att uppdatera dessa verksamheten framåtriktade kommentarer. Vi identifi erar framåtriktade I förvaltningsberättelsen och i avsnittet Utvecklingen i Risker beträffande framåtriktade kommentarer kommentarer genom ord som till exempel ”förutse”, sammandrag på sidorna 2 och 3 hänvisas till följande Syftet med denna årsredovisning med information från ”tror”, ”förväntar”, ”avser” och snarlika uttryck i sådana mått: Form 20-F är att tillhandahålla information till företagets kommentarer. -

30388 OID RS Annual Review
Review of the Year 2005/06 >> President’s foreword In the period covered by this review*, the Royal Society has continued and extended its activities over a wide front. There has, in particular, been an expansion in our international contacts and our engagement with global scientific issues. The joint statements on climate change and science in Africa, published in June 2005 by the science academies of the G8 nations, made a significant impact on the discussion before and at the Gleneagles summit. Following the success of these unprecedented statements, both of which were initiated by the Society, representatives of the science academies met at our premises in September 2005 to discuss how they might provide further independent advice to the governments of the G8. A key outcome of the meeting was an We have devoted increasing effort to nurturing agreement to prepare joint statements on the development of science academies overseas, energy security and infectious diseases ahead particularly in sub-Saharan Africa, and are of the St Petersburg summit in July 2006. building initiatives with academies in African The production of these statements, led by the countries through the Network of African Russian science academy, was a further Science Academies (NASAC). This is indicative illustration of the value of science academies of the long-term commitment we have made to working together to tackle issues of help African nations build their capacity in international importance. science, technology, engineering and medicine, particularly in universities and colleges. In 2004, the Society published, jointly with the Royal Academy of Engineering, a widely Much of the progress we have has made in acclaimed report on the potential health, recent years on the international stage has been environmental and social impacts of achieved through the tireless work of Professor nanotechnologies. -

Astrazeneca Notice of AGM 2007
NOTICE OF ANNUALAND SUMMARY GENERAL MEETINGFINANCIAL STATEMENT 2007 AND SHAREHOLDERS’ cIRCULAR WorldReginfo - 94cdbe6b-e6ef-4290-869e-95f1d4a336fd WorldReginfo - 94cdbe6b-e6ef-4290-869e-95f1d4a336fd LETTER FROM THE CHAIRMAN 1 LETTER FROM THE CHAIRMAN THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE AttENTION. IF YOU ARE or incurring because of the Political Parties, IN ANY DOUBT ABOUT ITS CONTENTS OR WHAT ACTION YOU SHOULD TAKE, YOU SHOULD Elections and Referendums Act 2000 in the CONSULT YOUR INDEPENDENT FINANCIAL ADVISER. IF YOU HAVE SOLD OR TRANSFERRED ALL UK (“the Act”). OF YOUR ASTRAZENECA ORDINARY SHARES YOU SHOULD SEND THIS DOCUMENT AND THE ACCOMPANYING DOCUMENTS TO THE PURCHASER OR TRANSFEREE OR TO THE STOCKBROKER, The Company has no intention of changing its BANK OR OTHER AGENT THROUGH WHOM THE SALE OR TRANSFER WAS EFFECTED FOR current practice of not making donations to TRANSMISSION TO THE PURCHASER OR TRANSFEREE. political parties in the EU and it will not do so without the specific endorsement of its shareholders. However, the Act defines DEAR SHAREHOLDER Senior Independent Director. Sir Peter was ‘political organisation’ widely so as to include, On behalf of the Board of AstraZeneca PLC, first appointed to the Board in January 1995. amongst other things, an organisation that I enclose various documents concerning your carries on activities that are capable of being shareholding in the Company. These are: On behalf of the whole Board, I would like reasonably regarded as intended to influence to express our gratitude to Erna and Sir public support for a political party in any EU 1 A Shareholders’ Circular incorporating Peter for their considerable contribution to member state or to influence voters in relation the formal Notice of the Annual General AstraZeneca’s success and wish them both to any referendum in any EU member state. -

Annual Report and Form 20-F Information 2003 AZ AR Innercovs.Qxd 18/2/04 3:58 PM Page 1
AZ_AR_OuterCovs.qxd 18/2/04 3:59 PM Page 1 AstraZeneca Annual Report and Form 20-F Information 2003 Annual Report and Form 20-F Information 2003 AZ_AR_InnerCovs.qxd 18/2/04 3:58 PM Page 1 AstraZeneca Annual Report and Contents Form 20-F Information 2003 Contents Cross Reference to The information in this document that is r Key Achievements 01 Financial Statements 21. Reserves 86 with the Securities and Exchange Comm Chairman’s Statement 02 Preparation of the Financial Statements 22. Net cash inflow from into any filings by AstraZeneca under the Global Market Overview 03 and Directors’ Responsibilities 60 trading operations 87 such major headings, including subhead Chief Executive’s Review 04 Basis of Consolidation and Presentation 23. Cash flows related Graphs are not included unless specifica Financial Highlights 05 of Financial Information 60 to exceptional items 87 SEC passed comment upon the accurac Board of Directors 06 Independent Auditor’s Report to the 24. Acquisitions of subsidiaries and information and may be updated from tim Members of AstraZeneca PLC 61 purchases of minority interests 87 Strategy 08 Group Profit and Loss Account 62 25. Disposals of business operations 88 Operational Review 09 Group Statement of Total Recognised 26. Reconciliation of net cash flow Cardiovascular 10 Item Gains and Losses 62 to movement in net funds 88 3 Key Information Gastrointestinal 12 Group Balance Sheet 64 27. Analysis of net funds 89 Neuroscience 14 A. Selected financial data Statement of Group Cash Flow 65 28. Financing 89 Financial Highlights Oncology 16 Accounting Policies 66 29. Post-retirement benefits 90 Group Financial Record Respiratory and Inflammation 18 Notes to the Financial Statements 30.