Shareholders' Circular PDF 1815KB
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Analyst Report
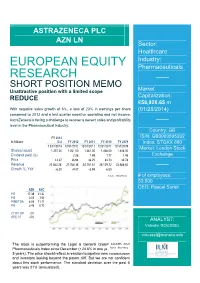
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -
View/Print Page As PDF
MENU Policy Analysis / Muqawama Fake News Surrounding Qasim Muslih’s Arrest (Part 2): Release Claims by Michael Knights, Crispin Smith, Hamdi Malik May 28, 2021 Also available in Arabic ABOUT THE AUTHORS Michael Knights Michael Knights is the Boston-based Jill and Jay Bernstein Fellow of The Washington Institute, specializing in the military and security affairs of Iraq, Iran, and the Persian Gulf states. Crispin Smith Crispin Smith is an associate at a Washington-based national security law group. His research focuses on Iraqi security, human rights, and law of armed conflict issues. Hamdi Malik Dr. Hamdi Malik is an Associate Fellow with the Washington Institute, specializing in Shia militias. He is the co-founder of the Militia Spotlight platform, which offers in-depth analysis of developments related to the Iranian-backed militias in Iraq and Syria. He is the coauthor of the Institute's 2020 study "Honored, Not Contained: The Future of Iraq’s Popular Mobilization Forces." Brief Analysis Part of a series: Militia Spotlight or see Part 1: How to Use Militia Spotlight Militias made a concerted effort to create the false impression that their colleague had been released immediately, and the claim was soon echoed by major global media outlets. n May 26, the Iraqi state arrested militia leader Qasim Muslih in connection with the May 9 murder of activist O Ehab al-Wazni. In June 2020, when fourteen Kataib Hezbollah (KH) members were arrested and thirteen suspects not covered by the warrant were quickly released, muqawama (resistance) groups were able to badly damage the Iraqi government’s credibility by publicizing the release. -

Truth in the Information Age
Beuth Hochschule für Technik Berlin Sprachenpreis 2019 Truth in the Information Age - Dead or Just Hidden? By Miriam Werner Theater- und Veranstaltungstechnik und -management 20.09.2019 Introduction Pokémon Go, iPhone 7, Donald Trump, Prince and Powerball. Those were the top five Google searches in 2016.1 Although it is hard to get a specific number from Google, it is estimated that Google handles more than two trillion searches per year. When doing the math that makes more than 63,000 searches per second.2 With numbers like these, you can see how important it is for us to gather information and with the right tools we have 24/7 access as well. If it didn’t matter what sort of information we receive, then everything would be fine. But in the era where expressions like “fake news” seem to pop up everywhere, the question seems to arise whether anything we read is true, or better yet, “is truth dead in the information age?” To fully understand all the nuances of this question a few definitions are required. When looking at the expression “information age” it refers to a period of time starting at the end of the 20th century and continuing into the foreseeable future. It is the change from the industrial to the post-industrial society where a large portion of the economy lies in information technology. It is shaped by the invention of the computer and telecommunication thereby changing how information is spread, managed and saved. 3 For this essay, I will be referring to the last three to four decades that include the invention of the internet, allowing information to travel around the world in seconds as well as offering access to more people than ever before. -

Affymetrix and the "DNA Chip" Revolution
taken pride in placing in the top 10 among the The Evolution of Astra best companies to work for in a number of ∗ magazine surveys, from Fortune to Working Merck Inc. in 1998 Mother. Merck & Co. sales grew from $9.6 billion in 1992 to $23 billion by the end of "We plan to revolutionize the pharmaceutical industry by 19972, topping the world drug sales league in being the best at linking patients and products.” Wayne Yetter, President of Astra Merck Inc. both years. In fact, Merck has seen its global market share jump one-third in the 1992-1998 period to edge Glaxo Wellcome in 1997 with a Section I. Background 3 4.6% world market share. In contrast to the In 1982, Merck & Co. and Astra AB signed an strategy of other pharmaceutical companies such agreement by which products resulting from as Glaxo, this growth was largely fueled by Astra’s research pipeline would be taken through internal R&D efforts as opposed to mergers and clinical trials and registration, to be ultimately acquisitions. marketed and sold by Merck in the United States. The initial terms of the contract specified Another major strategic move made to enhance that Astra’s products would be transferred to its competitive position was the acquisition of Merck’s development and marketing Medco Containment Services Inc. in 1993 organizations, with Astra receiving royalties on (renamed Merck-Medco Managed Care). the sales of the Astra drugs.1 Merck-Medco provides pharmaceutical benefit services in the United States to control The initial cooperation and relationship between prescription drug benefits cost. -

Fact and Fiction
ACTIVITY SHEET A Fact and Fiction There are many types of material produced in our digital age that are meant to persuade consumers, to sell products, or to exploit big issues—some more dangerous than others. An article that looks like news but is actually an advertisement for a new shopping mall is not as dangerous as an article that appears to be objective but is actually propaganda meant to influence a local election. Once you understand the varieties of non-news content and disinformation out there, you’re on your way to becoming an informed reader of news. INSTRUCTIONS Below, use what you learned in class to fill in the definition for each kind. Then, provide an example of that category of content that you have seen or read. If an example does not come to mind, imagine an example that would fit the description. DEFINITIONS EXAMPLES n Propaganda Example n Hoax Example n Agenda-Based Misrepresentation Example n Circular Reporting Example n Clickbait Example n Advertisements or Sponsored Content Example ACTIVITY SHEET B Be an Active News Consumer Spotting an artificial version of something can be hard. Counterfeit dollar bills are made to look identical to real currency. Pieces of forged art have been sold for millions of dollars. And spotting disinformation disguised to look like credible journalism can be tricky. At first, anyway. Fortunately, there are ways to look at news that help us tell serious journalism from other kinds of content. In the spaces below, paraphrase a piece of disinformation or non-news content and an example of credible journalism, both of which you’ll find online. -

CPC Outreach Journal #484
USAF COUNTERPROLIFERATION CENTER CPC OUTREACH JOURNAL Maxwell AFB, Alabama Issue No. 484, 21 February 2006 Articles & Other Documents: Commercial Photos Show Chinese Nuke Buildup Rice Asks For $75 Million To Increase Pressure On Iran Niger Uranium Rumors Wouldn't Die Iran Working On Nuclear Arms Plan, France Says Iran Hints At Compromise On Nuclear Inspections Europeans Reaffirm Diplomacy With Iran Bioterrorism, Hyped Iranian Fatwa Approves Use Of Nuclear Weapons Nuke-Armed Iran A Way Off: Experts On tape, Hussein talks of WMDs Talks On Enriching Nuclear Fuel For Iran In Russia Seem To Stall Welcome to the CPC Outreach Journal. As part of USAF Counterproliferation Center’s mission to counter weapons of mass destruction through education and research, we’re providing our government and civilian community a source for timely counterproliferation information. This information includes articles, papers and other documents addressing issues pertinent to US military response options for dealing with nuclear, biological and chemical threats and attacks. It’s our hope this information resource will help enhance your counterproliferation issue awareness. Established in 1998, the USAF/CPC provides education and research to present and future leaders of the Air Force, as well as to members of other branches of the armed services and Department of Defense. Our purpose is to help those agencies better prepare to counter the threat from weapons of mass destruction. Please feel free to visit our web site at www.au.af.mil/au/awc/awcgate/awc-cps.htm for in-depth information and specific points of contact. Please direct any questions or comments on CPC Outreach Journal to Jo Ann Eddy, CPC Outreach Editor, at (334) 953-7538 or DSN 493-7538. -

Download Current Issue
Global Security and Intelligence Studies Volume 5, Number 2 • Fall / Winter 2020 © 2020 Policy Studies Organization Editorial Welcome ......................................................................................... vii Melissa Layne and Carter Matherly Policy Relevant Commentary Prediction, Plus Patchwork, Equals Pandemic ................................................. 1 Margaret Marangione Research Articles Just Short of Cyberwar: A Focus on Jus Ad Vim to Inform an Ethical Framework for Cyberspace ............................................................ 19 Al Lewis The New River Report: Socio-Ecological System Impacts of Anthropogenic Pollution on New River Communities in Belize ................... 41 Kristin Drexler International NGOs Targeted by Terror: The Impact of Religiosity on Independence, Neutrality, and Impartiality .............................................. 73 Kathryn Lambert How Norm-Based Issue Frames Shape Public Support for Refugee Protection Policy: An Analysis Based on Survey Experiments in France and Germany ....................................................................................... 99 Melissa Schnyder Operationalizing Intelligence Collection in a Complex World: Bridging the Domestic & Foreign Intelligence Divide ................................. 123 Jim Burch (cont’d.) Notes from the Field Wrangling Stochasticity & Deconstructing Dimensionality: An Illustration of Fractals in Discursive Spaces ........................................... 155 Douglas Rose Book Reviews Mindf*ck, Cambridge -

ATP 2-33.4 Intelligence Analysis
ATP 2-33.4 Intelligence Analysis JANUARY 2020 DISTRIBUTION RESTRICTION: Approved for public release; distribution is unlimited. This publication supersedes ATP 2-33.4, dated 18 August 2014. Headquarters, Department of the Army This publication is available at Army Knowledge Online (https://armypubs.army.mil), and the Central Army Registry site (https://atiam.train.army.mil/catalog/dashboard). *ATP 2-33.4 Army Techniques Publication Headquarters No. 2-33.4 Department of the Army Washington, DC, 10 January 2020 Intelligence Analysis Contents Page PREFACE............................................................................................................. vii INTRODUCTION ................................................................................................... xi PART ONE FUNDAMENTALS Chapter 1 UNDERSTANDING INTELLIGENCE ANALYSIS ............................................. 1-1 Intelligence Analysis Overview ........................................................................... 1-1 Conducting Intelligence Analysis ........................................................................ 1-5 Intelligence Analysis and Collection Management ............................................. 1-8 The All-Source Intelligence Architecture and Analysis Across the Echelons ..... 1-9 Intelligence Analysis During Large-Scale Ground Combat Operations ........... 1-11 Intelligence Analysis During the Army’s Other Strategic Roles ........................ 1-13 Chapter 2 THE INTELLIGENCE ANALYSIS PROCESS .................................................. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

How to Sustain Healthcare Transformations
Voices on transformation A marathon, not a sprint: How to sustain healthcare transformations Voices on transformation is written by experts and practitioners in McKinsey & Company’s Pharmaceuticals and Medical Products Practice. To send comments or request copies of this publication, please email us at [email protected] Editors: Gayane Gyurjyan, Ioana Parsons, Shail Thaker, Jill Willder, Carla Zwaanstra Artwork and design: Afitha de Rijk-Voeten Portrait illustrations: Allan Burch Special thanks to: Lucia Darino, Martin Dewhurst, Claudio Feser, Vincent Forlenza, Jane Griffiths, Judith Hazlewood, Nadine Mansour, Angelika Reich, Pascal Soriot, David Speiser, Kirsten Westhues, André Wyss Copyright © 2016 McKinsey & Company. All rights reserved. This publication is not intended to be used as the basis for trading in the shares of any company or for undertaking any other complex or significant financial transaction without consulting appropriate professional advisers. No part of this publication may be copied or redistributed in any form without the prior written consent of McKinsey & Company. 2 PMP Voices on transformation <Chaper title> Contents Introduction 4 Cracking the code: How successful pharma leaders 7 manage transformations A health check for pharma: Overcoming change fatigue 15 in the pharmaceutical industry Putting science at the heart of renewed purpose 26 An interview with Pascal Soriot, AstraZeneca Transforming a medical devices company into a solutions provider 34 An interview with Vincent Forlenza, Becton Dickinson Refocusing the business around patient outcomes 42 An interview with Jane Griffiths, Janssen EMEA NBS: Creating value across Novartis 48 An interview with André Wyss, Novartis Voices on digital: How pharma can win in a digital world 57 About the authors 66 3 Introduction Over the past decade, the pharmaceutical industry has been struggling to keep up with rapid and dramatic changes in the external environment. -

1999 Annual Report and Form 20-F CONTENTS
1999 Annual Report and Form 20-F CONTENTS Key Achievements in 1999 1 Financial Highlights 2 Shareholder Highlights 5 Chairman’s Statement 6 Chief Executive’s Review 7 Operational Review 8 Safety, Health and Environment 32 In April 1999, Astra AB and Zeneca Group PLC People and Community 34 merged to form AstraZeneca, one of the world’s Financial Review 35 leading pharmaceutical and agrochemical Board of Directors and Officers companies, which provides innovative, effective of the Company 48 products to improve health, nutrition and quality Directors’ Report 49 of life worldwide. The company is research and Financial Statements 55 technology intensive, with extensive international Financial statements and notes development and marketing skills. Its healthcare relating to the financial statements 55 business is strategically focused on seven major Principal subsidiaries, joint ventures therapeutic areas: gastrointestinal, oncology, pain and associates 118 control and anaesthesia, cardiovascular, central Additional information for US investors 120 nervous system, respiratory and infection. Zeneca Group Financial Record 129 Agrochemicals provides crop protection products Shareholder Information 131 designed to improve crop yields and food quality. Exchange rates 138 Definitions 139 Glossary of Terms 140 Cross Reference to Form 20-F IBC Cautionary statement regarding forward-looking statements In order to utilise the ‘Safe Harbor’ provisions of the United States Private Securities Litigation Reform Act of 1995, AstraZeneca is providing the following cautionary statement. This Annual Report and Form 20-F 1999 contains forward-looking statements with respect to the financial condition, results of operations and businesses of AstraZeneca. By their nature, forward-looking statements and forecasts involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future. -

Bivictrix Therapeutics Plc (A Company Incorporated in England and Wales Under the Companies Act 2006 with Company Number 13470690)
THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION. If you are in any doubt about the contents of this document, you should consult your stockbroker, bank manager, solicitor, accountant or other independent professional adviser who specialises in advising on the acquisition of shares and other securities and is duly authorised under the Financial Services and Markets Act 2000 (as amended) (“FSMA”), if you are resident in the UK, or if you are not resident in the UK, from another appropriately authorised independent adviser. This document, which comprises an AIM admission document drawn up in accordance with the AIM Rules, has been issued in connection with the application for admission to trading on AIM of the entire issued and to be issued ordinary share capital of the Company. This document does not constitute an offer to the public requiring an approved prospectus under section 85 of FSMA and, accordingly, this document does not constitute a prospectus for the purposes of FSMA and the Prospectus Regulation Rules and has not been pre-approved by the FCA pursuant to section 85 of FSMA. Application has been made for the entire issued and to be issued ordinary share capital of the Company (the “Shares”) to be admitted to trading on AIM, a market operated by the London Stock Exchange. It is expected that Admission will become effective, and that dealings in the Shares will commence on 11 August 2021. The Shares are not dealt on any other recognised investment exchange and no application has been or is being made for the Shares to be admitted to any such exchange.