What Science Can Do
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -
Analyst Report
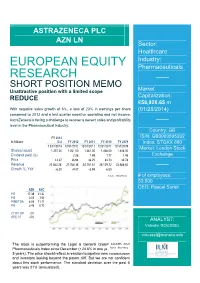
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Affymetrix and the "DNA Chip" Revolution
taken pride in placing in the top 10 among the The Evolution of Astra best companies to work for in a number of ∗ magazine surveys, from Fortune to Working Merck Inc. in 1998 Mother. Merck & Co. sales grew from $9.6 billion in 1992 to $23 billion by the end of "We plan to revolutionize the pharmaceutical industry by 19972, topping the world drug sales league in being the best at linking patients and products.” Wayne Yetter, President of Astra Merck Inc. both years. In fact, Merck has seen its global market share jump one-third in the 1992-1998 period to edge Glaxo Wellcome in 1997 with a Section I. Background 3 4.6% world market share. In contrast to the In 1982, Merck & Co. and Astra AB signed an strategy of other pharmaceutical companies such agreement by which products resulting from as Glaxo, this growth was largely fueled by Astra’s research pipeline would be taken through internal R&D efforts as opposed to mergers and clinical trials and registration, to be ultimately acquisitions. marketed and sold by Merck in the United States. The initial terms of the contract specified Another major strategic move made to enhance that Astra’s products would be transferred to its competitive position was the acquisition of Merck’s development and marketing Medco Containment Services Inc. in 1993 organizations, with Astra receiving royalties on (renamed Merck-Medco Managed Care). the sales of the Astra drugs.1 Merck-Medco provides pharmaceutical benefit services in the United States to control The initial cooperation and relationship between prescription drug benefits cost. -

Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories
bioRxiv preprint doi: https://doi.org/10.1101/572958; this version posted March 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Title: Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories. Authors: Konrad Krawczyk1*, Matthew Raybould2, Aleksandr Kovaltsuk2, Charlotte M. Deane2 1 NaturalAntibody, Hamburg, Germany 2 Oxford University Department of Statistics, Oxford, UK *Correspondence to [email protected] Abstract: Recently it has become possible to query the great diversity of natural antibody repertoires using Next Generation Sequencing (NGS). These methods are capable of producing millions of sequences in a single experiment. Here we compare Clinical Stage Therapeutic antibodies to the ~1b sequences from 60 independent sequencing studies in the Observed Antibody Space Database. Of the 242 post Phase I antibodies, we find 16 with sequence identity matches of 95% or better for both heavy and light chains. There are also 54 perfect matches to therapeutic CDR-H3 regions in the NGS outputs, suggesting a nontrivial amount of convergence between naturally observed sequences and those developed artificially. This has potential implications for both the discovery of antibody therapeutics and the legal protection of commercial antibodies. Introduction Antibodies are proteins in jawed vertebrates that recognize noxious molecules (antigens) for elimination. An organism expresses millions of diverse antibodies to increase the chances that some of them will be able to bind the foreign antigen, initiating the adaptive immune response. -

(12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub
US 20170172932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2017/0172932 A1 Peyman (43) Pub. Date: Jun. 22, 2017 (54) EARLY CANCER DETECTION AND A 6LX 39/395 (2006.01) ENHANCED IMMUNOTHERAPY A61R 4I/00 (2006.01) (52) U.S. Cl. (71) Applicant: Gholam A. Peyman, Sun City, AZ CPC .......... A61K 9/50 (2013.01); A61K 39/39558 (US) (2013.01); A61K 4I/0052 (2013.01); A61 K 48/00 (2013.01); A61K 35/17 (2013.01); A61 K (72) Inventor: sham A. Peyman, Sun City, AZ 35/15 (2013.01); A61K 2035/124 (2013.01) (21) Appl. No.: 15/143,981 (57) ABSTRACT (22) Filed: May 2, 2016 A method of therapy for a tumor or other pathology by administering a combination of thermotherapy and immu Related U.S. Application Data notherapy optionally combined with gene delivery. The combination therapy beneficially treats the tumor and pre (63) Continuation-in-part of application No. 14/976,321, vents tumor recurrence, either locally or at a different site, by filed on Dec. 21, 2015. boosting the patient’s immune response both at the time or original therapy and/or for later therapy. With respect to Publication Classification gene delivery, the inventive method may be used in cancer (51) Int. Cl. therapy, but is not limited to such use; it will be appreciated A 6LX 9/50 (2006.01) that the inventive method may be used for gene delivery in A6 IK 35/5 (2006.01) general. The controlled and precise application of thermal A6 IK 4.8/00 (2006.01) energy enhances gene transfer to any cell, whether the cell A 6LX 35/7 (2006.01) is a neoplastic cell, a pre-neoplastic cell, or a normal cell. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

How to Sustain Healthcare Transformations
Voices on transformation A marathon, not a sprint: How to sustain healthcare transformations Voices on transformation is written by experts and practitioners in McKinsey & Company’s Pharmaceuticals and Medical Products Practice. To send comments or request copies of this publication, please email us at [email protected] Editors: Gayane Gyurjyan, Ioana Parsons, Shail Thaker, Jill Willder, Carla Zwaanstra Artwork and design: Afitha de Rijk-Voeten Portrait illustrations: Allan Burch Special thanks to: Lucia Darino, Martin Dewhurst, Claudio Feser, Vincent Forlenza, Jane Griffiths, Judith Hazlewood, Nadine Mansour, Angelika Reich, Pascal Soriot, David Speiser, Kirsten Westhues, André Wyss Copyright © 2016 McKinsey & Company. All rights reserved. This publication is not intended to be used as the basis for trading in the shares of any company or for undertaking any other complex or significant financial transaction without consulting appropriate professional advisers. No part of this publication may be copied or redistributed in any form without the prior written consent of McKinsey & Company. 2 PMP Voices on transformation <Chaper title> Contents Introduction 4 Cracking the code: How successful pharma leaders 7 manage transformations A health check for pharma: Overcoming change fatigue 15 in the pharmaceutical industry Putting science at the heart of renewed purpose 26 An interview with Pascal Soriot, AstraZeneca Transforming a medical devices company into a solutions provider 34 An interview with Vincent Forlenza, Becton Dickinson Refocusing the business around patient outcomes 42 An interview with Jane Griffiths, Janssen EMEA NBS: Creating value across Novartis 48 An interview with André Wyss, Novartis Voices on digital: How pharma can win in a digital world 57 About the authors 66 3 Introduction Over the past decade, the pharmaceutical industry has been struggling to keep up with rapid and dramatic changes in the external environment. -

Lääkeaineiden Yleisnimet (INN-Nimet) 21.6.2021
Lääkealan turvallisuus- ja kehittämiskeskus Säkerhets- och utvecklingscentret för läkemedelsområdet Finnish Medicines Agency Lääkeaineiden yleisnimet (INN-nimet) 21.6. -

1999 Annual Report and Form 20-F CONTENTS
1999 Annual Report and Form 20-F CONTENTS Key Achievements in 1999 1 Financial Highlights 2 Shareholder Highlights 5 Chairman’s Statement 6 Chief Executive’s Review 7 Operational Review 8 Safety, Health and Environment 32 In April 1999, Astra AB and Zeneca Group PLC People and Community 34 merged to form AstraZeneca, one of the world’s Financial Review 35 leading pharmaceutical and agrochemical Board of Directors and Officers companies, which provides innovative, effective of the Company 48 products to improve health, nutrition and quality Directors’ Report 49 of life worldwide. The company is research and Financial Statements 55 technology intensive, with extensive international Financial statements and notes development and marketing skills. Its healthcare relating to the financial statements 55 business is strategically focused on seven major Principal subsidiaries, joint ventures therapeutic areas: gastrointestinal, oncology, pain and associates 118 control and anaesthesia, cardiovascular, central Additional information for US investors 120 nervous system, respiratory and infection. Zeneca Group Financial Record 129 Agrochemicals provides crop protection products Shareholder Information 131 designed to improve crop yields and food quality. Exchange rates 138 Definitions 139 Glossary of Terms 140 Cross Reference to Form 20-F IBC Cautionary statement regarding forward-looking statements In order to utilise the ‘Safe Harbor’ provisions of the United States Private Securities Litigation Reform Act of 1995, AstraZeneca is providing the following cautionary statement. This Annual Report and Form 20-F 1999 contains forward-looking statements with respect to the financial condition, results of operations and businesses of AstraZeneca. By their nature, forward-looking statements and forecasts involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future. -

Astrazeneca Annual Report 2006
AND FORM 20-F ANNUAL INFORMATION REPORT 2006 CONTENTS 2006 IN BRIEF 1 FINANCIAL STATEMENTS 19. Reserves 120 CHAIRMAN’S STATEMENT 2 Preparation of the Financial Statements 20. Minority interests 121 CHIEF EXECUTIVE OFFICER’S REVIEW 3 and Directors’ Responsibilities 96 21. Dividends to shareholders 121 FINANCIAL HIGHLIGHTS 6 Directors’ Responsibilities for, and Report 22. Acquisitions of on, Internal Control over Financial DIRECTORS’ REPORT business operations 121 Reporting 96 Business review 8 23. Disposal of business operations 123 Auditors’ Reports on the Financial 24. Post-retirement benefi ts 123 > Business environment 9 Statements and on Internal Control over > Strategy 11 Financial Reporting (Sarbanes-Oxley Act 25. Employee costs and share option plans for employees 128 > Our resources, skills Section 404) 97 and capabilities 12 Independent Auditors’ Report to the 26. Commitments and contingent liabilities 133 > Measuring performance 15 Members of AstraZeneca PLC (Group) 97 27. Leases 146 > Therapy area review Consolidated Income Statement 98 28. Statutory and other information 146 – Cardiovascular medicines 16 Consolidated Statement of Recognised Income and Expense 98 29. Share capital of parent – Gastrointestinal medicines 20 Consolidated Balance Sheet 99 company 147 – Neuroscience medicines 23 Consolidated Cash Flow Statement 100 Principal Subsidiaries 148 – Oncology medicines 26 Accounting Policies (Group) 101 Additional Information for US Investors 149 – Respiratory and Independent Auditors’ Infl ammation medicines 29 Notes to the Financial Statements (Group) Report to the Members of – Infection medicines 32 AstraZeneca PLC (Company) 157 1. Operating profi t 104 > Geographic review 33 Company Balance Sheet 158 2. Profi t on sale of interest > Research and development 37 in joint venture 104 Accounting Policies (Company) 159 > Development pipeline table 40 3. -

Astrazeneca: the First Abuse Case in the Pharmaceutical Sector Niklas FAGERLUND and Søren Bo RASMUSSEN, Directorate-General Competition, Unit B-2
Antitrust AstraZeneca: the first abuse case in the pharmaceutical sector Niklas FAGERLUND and Søren Bo RASMUSSEN, Directorate-General Competition, unit B-2 1. Introduction by AZ before patent offices in Belgium, Denmark, Germany, the Netherlands, Norway and the United On 15 June 2005 the Commission adopted a deci- Kingdom and before national courts in Germany sion (‘Decision’) fining the Swedish company and Norway. AstraZeneca AB and the UK company Astra- Zeneca Plc (together ‘AZ’) 60 million euros due to The misleading information was provided by AZ their infringements of Article 82 of the EC Treaty in the context of its two rounds of applications and Article 54 of the EEA Agreement. (in June 1993 and December 1994) to several pat- ent offices within the EEA for extra protection for The infringements involve misuses by AZ of public omeprazole (the active substance in AZ’s product procedures and regulations in a number of EEA Losec) in the form of so-called supplementary states aimed at excluding generic firms and paral- protection certificates (SPCs). Under the SPC Reg- lel traders from competing against AZ’s anti-ulcer ulation the basic patent protection for active sub- product Losec. stances in medicines can be extended by a maxi- In 1979, Astra AB (currently AstraZeneca AB), a mum of five years. Swedish research based company, had filed pat- The Decision raises no objections to AZ’s incorrect ent applications in Europe in respect of omepra- interpretation of the relevant legislation. Therefore, zole (the active substance in Losec). Losec’s basic the proceedings and outcome in Hässle AB v.