2007English.87123248
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Otonomy Inc. 2021 Proxy Statement
OTONOMY, INC. Dear Stockholder: I am pleased to invite you to attend the 2021 Annual Meeting of Stockholders (the “Annual Meeting”) of Otonomy, Inc. (“Otonomy”), which will be held on June 22, 2021 at 8:00 a.m. Pacific Time. The Annual Meeting will be conducted virtually via live webcast. You will be able to attend the Annual Meeting by visiting www.virtualshareholdermeeting.com/OTIC2021, where you will be able to listen to the meeting live, submit questions and vote online by entering the control number located on your proxy card. The attached Notice of Annual Meeting of Stockholders and proxy statement contain details of the business to be conducted at the Annual Meeting. Whether or not you attend the Annual Meeting, it is important that your shares be represented and voted at the meeting. Therefore, I urge you to promptly vote and submit your proxy via the Internet, by phone, or by signing, dating and returning the enclosed proxy card in the enclosed envelope. If you decide to attend the Annual Meeting, you will be able to change your vote or revoke your proxy, even if you have previously submitted your proxy. On behalf of Otonomy, I would like to thank you for your continued support. Sincerely, David A. Weber, Ph.D. President and Chief Executive Officer OTONOMY, INC. 4796 Executive Drive San Diego, California 92121 NOTICE OF ANNUAL MEETING OF STOCKHOLDERS Time and Date June 22, 2021 at 8:00 a.m. Pacific Time The Annual Meeting will be a completely virtual meeting of stockholders, to be conducted via live webcast. -

Chief Executive Officer Graham Baker
OVERVIEW OUR BUSINESS OPERATIONAL FINANCIAL RISK GOVERNANCE ACCOUNTS 48 & MARKETPLACE REVIEW REVIEW OUR BOARD OF DIRECTORS ROBERTO QUARTA (67) OLIVIER BOHUON (58) GRAHAM BAKER (48) CHAIRMAN CHIEF EXECUTIVE OFFICER CHIEF FINANCIAL OFFICER Joined the Board in December 2013 and Joined the Board and was appointed Chief -RLQLQJWKH%RDUGDV&KLHI)LQDQFLDO2I²FHULQ appointed Chairman following election by ([HFXWLYH2I²FHULQ$SULO+HUHVLJQHGDV March 2017. shareholders at the April 2014 Annual General a Member of the Nomination & Governance Meeting. He was also appointed Chairman of &RPPLWWHHRQ)HEUXDU\ CAREER AND EXPERIENCE the Nomination & Governance Committee and Graham holds an MA degree in Economics from a Member of the Remuneration Committee on CAREER AND EXPERIENCE Cambridge University and qualified as a Chartered that day. Olivier holds a doctorate in Pharmacy from the Accountant and Chartered Tax Advisor with Arthur University of Paris and an MBA from HEC, Paris. Andersen. In 1995, he joined AstraZeneca PLC where CAREER AND EXPERIENCE He started his career in Morocco with Roussel Uclaf he worked for 20 years, holding multiple senior roles, Roberto is a graduate and a former Trustee of the S.A. and then, with the same company, held a number including Vice President, Finance, International (2013- College of the Holy Cross, Worcester (MA), US. of positions in the Middle East with increasing levels of 2015) with responsibility for all emerging markets, He started his career as a manager trainee at David responsibility. He joined Abbott in Chicago as head of Vice President, Global Financial Services (2011-2013) Gessner Ltd, before moving on to Worcester Controls their anti-infective franchise with Abbott International and Vice President Finance & Chief Financial Officer, Corporation and then BTR plc, where he was a before becoming Pharmaceutical General Manager North America (2008-10). -
Analyst Report
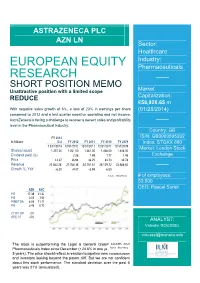
ASTRAZENECA PLC AZN LN Sector: Healthcare Industry: EUROPEAN EQUITY Pharmaceuticals RESEARCH I SHORT POSITION MEMO Market Unattractive position with a limited scope REDUCE Capitalization: €58,920.65 m With negative sales growth of 6 %, a loss of 23% in earnings per share (01/28/2014) compared to 2012 and a last quarter negative operating and net income, AstraZeneca is facing a challenge to recover a decent sales and profitability level in the Pharmaceutical industry. Country: GB FY 2013 ISIN: GB0009895292 In Millions Est FY 2012 FY 2011 FY 2010 FY 2009 Index: STOXX 600 12/31/2013 12/31/2012 12/31/2011 12/31/2010 12/31/2009 Market: London Stock Shares issued 1,257.00 1,261.00 1,361.00 1,438.00 1,448.00 Dividend yield (%) Exchange 2.26 1.99 1.77 1.48 Price 43.07 36.94 36.25 34.73 32.78 Revenue 20,462.28 21,768.38 24,151.51 25,129.22 23,588.60 oji Growth %, YoY -6.00 -9.87 -3.89 6.53 Source : Bloomberg # of employees: 53,500 CEO: Pascal Soriot AZN AVG P/E 17.38 21.32 P/S 3.05 3.56 P/EBITDA 8.04 11.11 P/B 3.45 5.72 2Y RV GR -2% OPE CF -5% ANALYST: Valentin ROUSSEL [email protected] The stock is outperforming the Legal & General Global Health and Pharmaceuticals Index since December (+24.6% in one year,Source +17.8% : Bloomberg in 5 years). The price should reflect a reaction to pipeline new introductions and investors looking beyond the patent cliff. -

Nexium Control, Esomeprazole
27 June 2013 EMA/498929/2013 Committee for Medicinal Products for Human Use (CHMP) Assessment report Nexium Control International non-proprietary name: esomeprazole Procedure No. EMEA/H/C/002618 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. 7 Westferry Circus ● Canary Wharf ● London E14 4HB ● United Kingdom Telephone +44 (0)20 7418 8400 Facsimile +44 (0)20 7418 8613 E -mail [email protected] Website www.ema.europa.eu An agency of the European Union © European Medicines Agency, 2013. Reproduction is authorised provided the source is acknowledged. Product information Marketing authorisation application Name of the medicinal product: Nexium Control Applicant: AstraZeneca AB Building 411A, Floor 4 S - 151 85 Södertälje SWEDEN Active substance: esomeprazole (as magnesium trihydrate) International Nonproprietary Name/Common Name: esomeprazole Pharmaco-therapeutic group Proton pump inhibitors (ATC Code): (A02BC05) Therapeutic indication: Nexium Control is indicated for the short-term treatment of reflux symptoms (e.g. heartburn and acid regurgitation) in adults. Pharmaceutical form: Gastro-resistant tablet Strength: 20 mg Route of administration: Oral use Packaging: blister (PVC/PVDC) Package sizes: 7 tablets and 14 tablets Assessment report EMA/498929/2013 Page 2/70 Table of contents 1. Background information on the procedure .............................................. 6 1.1. Submission of the dossier ..................................................................................... -

Affymetrix and the "DNA Chip" Revolution
taken pride in placing in the top 10 among the The Evolution of Astra best companies to work for in a number of ∗ magazine surveys, from Fortune to Working Merck Inc. in 1998 Mother. Merck & Co. sales grew from $9.6 billion in 1992 to $23 billion by the end of "We plan to revolutionize the pharmaceutical industry by 19972, topping the world drug sales league in being the best at linking patients and products.” Wayne Yetter, President of Astra Merck Inc. both years. In fact, Merck has seen its global market share jump one-third in the 1992-1998 period to edge Glaxo Wellcome in 1997 with a Section I. Background 3 4.6% world market share. In contrast to the In 1982, Merck & Co. and Astra AB signed an strategy of other pharmaceutical companies such agreement by which products resulting from as Glaxo, this growth was largely fueled by Astra’s research pipeline would be taken through internal R&D efforts as opposed to mergers and clinical trials and registration, to be ultimately acquisitions. marketed and sold by Merck in the United States. The initial terms of the contract specified Another major strategic move made to enhance that Astra’s products would be transferred to its competitive position was the acquisition of Merck’s development and marketing Medco Containment Services Inc. in 1993 organizations, with Astra receiving royalties on (renamed Merck-Medco Managed Care). the sales of the Astra drugs.1 Merck-Medco provides pharmaceutical benefit services in the United States to control The initial cooperation and relationship between prescription drug benefits cost. -

Esomeprazole: a New Proton Pump Inhibitor for NSAID-Associated Peptic Ulcers and Dyspepsia
DRUG PROFILE Esomeprazole: a new proton pump inhibitor for NSAID-associated peptic ulcers and dyspepsia Grace Lai-Hung Wong Ulcer and ulcer symptoms related to the use of non-steroidal anti-inflammatory drugs & Joseph JY Sung† (NSAIDs) and aspirin constitute a major global health issue. Despite various attempts to †Author for correspondence prevent and heal injuries inflicted by NSAIDs and aspirin, acid suppression remains one of The Prince of Wales Hospital, Department of Medicine and the cornerstones in the management of NSAID-associated ulcers. Esomeprazole, the Therapeutics, 9/F, S-optical isomer (enantiomer) of omeprazole, suppresses gastric acid secretion by 30–32 Ngan Shing Road, inhibiting the parietal cell membrane enzyme H+/K+-ATPase. With improved bioavailability, Shatin, NT, Hong Kong SAR due to reduced first-pass metabolism in the liver, esomeprazole promises to be more Tel.: +852 2632 3132 potent in acid suppression in the stomach. Similar to omeprazole, the safety profile of Fax: +852 2645 1699 esomeprazole has been well established. Clinical studies comparing esomeprazole with [email protected] other proton pump inhibitors (PPIs) in the healing of NSAID-related ulcer are few. Recent multicenter randomized studies demonstrated that esomeprazole significantly improves dyspeptic symptoms in patients taking nonselective NSAIDs and specific cyclooxygenase-2 inhibitors. Esomeprazole also protects the stomach from aspirin-induced ulcer bleeding. Safety profiles of esomeprazole appear promising. Non-steroidal anti-inflammatory drugs both basal and stimulated acid secretion. PPIs are (NSAIDs) are the most commonly used medica- most effective against meal-induced gastric acid tions for chronic pain and arthritis due to their secretion [3]. -

Dr. Reddy's – Recall of Ranitidine
Dr. Reddy’s – Recall of ranitidine • On October 23, 2019, Dr. Reddy’s announced the voluntary, consumer-level recall of prescription ranitidine due to potential contamination with N-nitrosodimethylamine (NDMA). • Dr. Reddy’s initially announced retail level recalls for prescription and OTC ranitidine in early October; however, the recall of prescription ranitidine products has been escalated to the consumer level. The recall of OTC ranitidine remains at the retail level. Prescription Ranitidine Capsules Recalled by Dr. Reddy’s Product Description NDC# Ranitidine 150 mg capsules, 60 count bottle 55111-129-60 Ranitidine 150 mg capsules, 500 count bottle 55111-129-05 Ranitidine 300 mg capsules, 30 count bottle 55111-130-30 Ranitidine 300 mg capsules, 100 count bottle 55111-130-01 • Refer to the Dr. Reddy’s announcement for a complete list of the OTC recalled ranitidine products. • Other manufacturers, including Apotex, Perrigo and Sanofi have recently announced retail level recalls of their OTC ranitidine products. • In September, Sandoz issued a consumer level recall of prescription ranitidine products. • These recalls follow a recent FDA statement about NDMA impurities detected in ranitidine medicines. • NDMA is classified as a probable human carcinogen based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables. • Ranitidine is an OTC and prescription drug. Ranitidine is an H2 (histamine-2) blocker, which decreases the amount of acid created by the stomach. OTC ranitidine is approved to prevent and relieve heartburn associated with acid ingestion and sour stomach. • Prescription ranitidine is approved for multiple indications, including treatment and prevention of ulcers of the stomach and intestines and treatment of gastroesophageal reflux disease. -

Pharmaceuticals, Medical Devices and Biologics Regulatory and Policy Bulletin
Antitrust Connection Spring 2009 To: Our Clients and Friends August 27, 2010 Pharmaceuticals, Medical Devices and Biologics Regulatory and Policy Bulletin Top News During China Visit, FDA Commissioner’s Focus Not on Heparin Investigation FDA Commissioner Margaret Hamburg’s recent trip to China focused primarily on avenues of collaboration and cooperation between the FDA and Chinese regulators rather than on the current investigation into the contamination of heparin, which has caused some to express concern that US legislators may perceive the two bodies as not trying to work effectively to determine the cause of the contamination. FDA officials have indicated that the issue was discussed, but it was not focused on during the meetings. Industry Expresses Concern Over New Drug User Fees The drug industry is expressing concern over the FDA’s recent increase in prescription drug user fee rates, saying that the increase of approximately 10 percent is not appropriate given the backlog and failure to meet other review deadlines. Industry has indicated that it would like more accountability and adherence to performance goals if will be paying such increased fees. As the FDA discusses drug user fees and other programs as part of the Prescription Drug User Fee Act legislative proposal, some consumer, safety, and patient groups are voicing their concern that the agency is not including them in its discussions of the Act. Official, Panel Members Criticize Glaxo’s Report on Avandia Committee Meeting An article in the New York Times indicates that a government official and some members of the FDA advisory panel that recently reviewed Avandia are critical of Glaxo’s letter to doctors participating in the TIDE trial, intended to compare the risks of Avandia with those of competing drug Actos. -

Label Phase of the Study
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ------------------------WARNINGS AND PRECAUTIONS---------------- NEXIUM safely and effectively. See full prescribing information for • Symptomatic response does not preclude the presence of gastric NEXIUM. malignancy (5.1) • Atrophic gastritis has been noted with long-term omeprazole therapy NEXIUM (esomeprazole magnesium) Delayed-Release Capsules, for Oral (5.2) Use • PPI therapy may be associated with increased risk of Clostridium NEXIUM (esomeprazole magnesium) For Delayed-Release Oral difficile associated diarrhea. (5.3) Suspension • Bone Fracture: Long-term and multiple daily dose PPI therapy may be Initial U.S. Approval: 1989 (omeprazole) associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. (5.4) -----------------------RECENT MAJOR CHANGES----------------------- • Hypomagnesemia has been reported rarely with prolonged treatment Warnings and Precautions, Clostridium difficile associated 09/2012 with PPIs (5.5) diarrhea (5.3) • Avoid concomitant use of NEXIUM with St John’s Wort or rifampin Warnings and Precautions, Concomitant use of NEXIUM with due to the potential reduction in esomeprazole levels (5.6) (7.3) Methotrexate (5.10) 01/2012 • Interactions with diagnostic investigations for Neuroendocrine Tumors: Increases in intragastric pH may result in hypergastrinemia and -----------------------INDICATIONS AND USAGE----------------------- enterochromaffin-like cell hyperplasia and -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

How to Sustain Healthcare Transformations
Voices on transformation A marathon, not a sprint: How to sustain healthcare transformations Voices on transformation is written by experts and practitioners in McKinsey & Company’s Pharmaceuticals and Medical Products Practice. To send comments or request copies of this publication, please email us at [email protected] Editors: Gayane Gyurjyan, Ioana Parsons, Shail Thaker, Jill Willder, Carla Zwaanstra Artwork and design: Afitha de Rijk-Voeten Portrait illustrations: Allan Burch Special thanks to: Lucia Darino, Martin Dewhurst, Claudio Feser, Vincent Forlenza, Jane Griffiths, Judith Hazlewood, Nadine Mansour, Angelika Reich, Pascal Soriot, David Speiser, Kirsten Westhues, André Wyss Copyright © 2016 McKinsey & Company. All rights reserved. This publication is not intended to be used as the basis for trading in the shares of any company or for undertaking any other complex or significant financial transaction without consulting appropriate professional advisers. No part of this publication may be copied or redistributed in any form without the prior written consent of McKinsey & Company. 2 PMP Voices on transformation <Chaper title> Contents Introduction 4 Cracking the code: How successful pharma leaders 7 manage transformations A health check for pharma: Overcoming change fatigue 15 in the pharmaceutical industry Putting science at the heart of renewed purpose 26 An interview with Pascal Soriot, AstraZeneca Transforming a medical devices company into a solutions provider 34 An interview with Vincent Forlenza, Becton Dickinson Refocusing the business around patient outcomes 42 An interview with Jane Griffiths, Janssen EMEA NBS: Creating value across Novartis 48 An interview with André Wyss, Novartis Voices on digital: How pharma can win in a digital world 57 About the authors 66 3 Introduction Over the past decade, the pharmaceutical industry has been struggling to keep up with rapid and dramatic changes in the external environment. -

1999 Annual Report and Form 20-F CONTENTS
1999 Annual Report and Form 20-F CONTENTS Key Achievements in 1999 1 Financial Highlights 2 Shareholder Highlights 5 Chairman’s Statement 6 Chief Executive’s Review 7 Operational Review 8 Safety, Health and Environment 32 In April 1999, Astra AB and Zeneca Group PLC People and Community 34 merged to form AstraZeneca, one of the world’s Financial Review 35 leading pharmaceutical and agrochemical Board of Directors and Officers companies, which provides innovative, effective of the Company 48 products to improve health, nutrition and quality Directors’ Report 49 of life worldwide. The company is research and Financial Statements 55 technology intensive, with extensive international Financial statements and notes development and marketing skills. Its healthcare relating to the financial statements 55 business is strategically focused on seven major Principal subsidiaries, joint ventures therapeutic areas: gastrointestinal, oncology, pain and associates 118 control and anaesthesia, cardiovascular, central Additional information for US investors 120 nervous system, respiratory and infection. Zeneca Group Financial Record 129 Agrochemicals provides crop protection products Shareholder Information 131 designed to improve crop yields and food quality. Exchange rates 138 Definitions 139 Glossary of Terms 140 Cross Reference to Form 20-F IBC Cautionary statement regarding forward-looking statements In order to utilise the ‘Safe Harbor’ provisions of the United States Private Securities Litigation Reform Act of 1995, AstraZeneca is providing the following cautionary statement. This Annual Report and Form 20-F 1999 contains forward-looking statements with respect to the financial condition, results of operations and businesses of AstraZeneca. By their nature, forward-looking statements and forecasts involve risk and uncertainty because they relate to events and depend on circumstances that will occur in the future.