Quite a Few Reasons for Calling Carnivores 'The Most Wonderful
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carnivorous Plant Responses to Resource Availability
Carnivorous plant responses to resource availability: environmental interactions, morphology and biochemistry Christopher R. Hatcher A doctoral thesis submitted in partial fulfilment of requirements for the award of Doctor of Philosophy of Loughborough University November 2019 © by Christopher R. Hatcher (2019) Abstract Understanding how organisms respond to resources available in the environment is a fundamental goal of ecology. Resource availability controls ecological processes at all levels of organisation, from molecular characteristics of individuals to community and biosphere. Climate change and other anthropogenically driven factors are altering environmental resource availability, and likely affects ecology at all levels of organisation. It is critical, therefore, to understand the ecological impact of environmental variation at a range of spatial and temporal scales. Consequently, I bring physiological, ecological, biochemical and evolutionary research together to determine how plants respond to resource availability. In this thesis I have measured the effects of resource availability on phenotypic plasticity, intraspecific trait variation and metabolic responses of carnivorous sundew plants. Carnivorous plants are interesting model systems for a range of evolutionary and ecological questions because of their specific adaptations to attaining nutrients. They can, therefore, provide interesting perspectives on existing questions, in this case trait-environment interactions, plant strategies and plant responses to predicted future environmental scenarios. In a manipulative experiment, I measured the phenotypic plasticity of naturally shaded Drosera rotundifolia in response to disturbance mediated changes in light availability over successive growing seasons. Following selective disturbance, D. rotundifolia became more carnivorous by increasing the number of trichomes and trichome density. These plants derived more N from prey and flowered earlier. -

Florida Council of Bromeliad Societies, Inc
Florida Council of Bromeliad Societies, Inc. In This Issue: 2007 Shows and Sales Cold Hardy Bromeliads List Vol. 27 Issue 1 February 2007 FCBS Affiliated Societies and Representatives B. Guild Tampa Bay Caloosahatchee Tom Wolfe Vicky Chirnside 5211 Lake LeClare Road 951 Southland Road Lutz 33558 Venice 34293 813-961-1475 941-493-5825 [email protected] [email protected] Bob Teems Tom Foley 813-855-0938 239-458-4656 Broward County Fl. East Coast Jose Donayre Calandra Thurrott 1240 Jefferson St. 713 Breckenridge Drive Hollywood 33019-1807 Port Orange 32127 954-925-5112 386-761-4804 Jcadonayre @bellsouth.net [email protected] Colleen Hendrix Carolyn Schoenau 954-530-0076 352-372-6589 Central Florida F. West Coast Betsy McCrory Linda Sheetz 3615 Boggy Creek Rd. 1153 Williams Dr. S Kissimmee 34744 St. Petersburg 33705 407-348-2139 727-864-3165 [email protected] [email protected] Butch Force Brian Corey 407-886-4814 727-864-3165 South Florida Gainesville Juan Espinosa-Almodovar Al Muzzell P.O. Box 430722 P.O. Box 14442 Miami 33243 Gainesville 32604 305-667-6155 352-372-4576 [email protected] John R. Moxley Michael Michalski 352-528-0783 305-279-2416 (Continued on the inside back cover.) 2007 Bromeliad Extravaganza Presented by Florida Council of Bromeliad Societies Hosted by the Bromeliad Society of Broward County Saturday, September 29, 2007 at the Hilton Ft. Lauderdale Airport Hotel 1870 Griffin Rd. Dania Beach, FL 33004 954-920-3300 954-920-3348 (fax) Room rates: Single or double $89.00 Rates in effect until September 14, 2007 Sale, Banquet, Raffle and Rare Plant Auction will take place at the same location. -
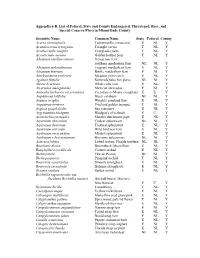
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Checklist Das Spermatophyta Do Estado De São Paulo, Brasil
Biota Neotrop., vol. 11(Supl.1) Checklist das Spermatophyta do Estado de São Paulo, Brasil Maria das Graças Lapa Wanderley1,10, George John Shepherd2, Suzana Ehlin Martins1, Tiago Egger Moellwald Duque Estrada3, Rebeca Politano Romanini1, Ingrid Koch4, José Rubens Pirani5, Therezinha Sant’Anna Melhem1, Ana Maria Giulietti Harley6, Luiza Sumiko Kinoshita2, Mara Angelina Galvão Magenta7, Hilda Maria Longhi Wagner8, Fábio de Barros9, Lúcia Garcez Lohmann5, Maria do Carmo Estanislau do Amaral2, Inês Cordeiro1, Sonia Aragaki1, Rosângela Simão Bianchini1 & Gerleni Lopes Esteves1 1Núcleo de Pesquisa Herbário do Estado, Instituto de Botânica, CP 68041, CEP 04045-972, São Paulo, SP, Brasil 2Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas – UNICAMP, CP 6109, CEP 13083-970, Campinas, SP, Brasil 3Programa Biota/FAPESP, Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas – UNICAMP, CP 6109, CEP 13083-970, Campinas, SP, Brasil 4Universidade Federal de São Carlos – UFSCar, Rod. João Leme dos Santos, Km 110, SP-264, Itinga, CEP 18052-780, Sorocaba, SP, Brasil 5Departamento de Botânica – IBUSP, Universidade de São Paulo – USP, Rua do Matão, 277, CEP 05508-090, Cidade Universitária, Butantã, São Paulo, SP, Brasil 6Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana – UEFS, Av. Transnordestina, s/n, Novo Horizonte, CEP 44036-900, Feira de Santana, BA, Brasil 7Universidade Santa Cecília – UNISANTA, R. Dr. Oswaldo Cruz, 266, Boqueirão, CEP 11045-907, -

FINAL REPORT PSRA Vegetation Monitoring 2005-2006 PC P502173
Rare Plants and Their Locations at Picayune Strand Restoration Area: Task 4a FINAL REPORT PSRA Vegetation Monitoring 2005-2006 PC P502173 Steven W. Woodmansee and Michael J. Barry [email protected] December 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to Mike Duever, Ph.D. Senior Environmental Scientist South Florida Water Management District Fort Myers Service Center 2301 McGregor Blvd. Fort Myers, Florida 33901 Table of Contents Introduction 03 Methods 03 Results and Discussion 05 Acknowledgements 38 Citations 39 Tables: Table 1: Rare plants recorded in the vicinity of the Vegetation Monitoring Transects 05 Table 2: The Vascular Plants of Picayune Strand State Forest 24 Figures: Figure 1: Picayune Strand Restoration Area 04 Figure 2: PSRA Rare Plants: Florida Panther NWR East 13 Figure 3: PSRA Rare Plants: Florida Panther NWR West 14 Figure 4: PSRA Rare Plants: PSSF Northeast 15 Figure 5: PSRA Rare Plants: PSSF Northwest 16 Figure 6: PSRA Rare Plants: FSPSP West 17 Figure 7: PSRA Rare Plants: PSSF Southeast 18 Figure 8: PSRA Rare Plants: PSSF Southwest 19 Figure 9: PSRA Rare Plants: FSPSP East 20 Figure 10: PSRA Rare Plants: TTINWR 21 Cover Photo: Bulbous adder’s tongue (Ophioglossum crotalophoroides), a species newly recorded for Collier County, and ranked as Critically Imperiled in South Florida by The Institute for Regional Conservation taken by the primary author. 2 Introduction The South Florida Water Management District (SFWMD) plans on restoring the hydrology at Picayune Strand Restoration Area (PSRA) see Figure 1. -

Natural Forest Community Delineation Methods
Natural Forest Community Delineation Methods Keith A. Bradley and George D. Gann February 2, 2005 The Institute for Regional Conservation 22601 S.W. 152 Avenue; Miami, Florida 33170 George D. Gann, Executive Director Introduction The Natural Forest Community (NFC) system was established in 1984 under ordinance 89-9, Chapter 24-60 of the Miami-Dade County Code. The ordinance provides legal protection for sites designated by the county as NFCs: “Natural Forest Community shall mean all stands of trees (including their associated understory) which were designated as Natural Forest Communities on the Dade County Natural Forest Community Maps and approved by the Board of County Commissioners, pursuant to Resolution No. R-1764-84.” Factors for reviewing proposed Natural Forest Community sites in the original ordinance included: 1) Presence of endangered, threatened, rare, or endemic species (plants or animals); 2) Plant species diversity on the site; 3) Size of trees; 4) Size of site; 5) Wildlife habitat value; 6) Geological features; and 7) Percentage of site covered by non-native plant species. As required by the ordinance, quantitative evaluation criteria were developed by DERM incorporating the above factors, including separate criteria for the delineation of hardwood hammocks and pinelands. These criteria have become outdated as more scientifically rigorous criteria for delineating natural areas have been developed since 1984, primarily relating to delineation of wetland habitats. Some of the methods used to delineate NFCs by DERM proved to be ineffective including the establishment of transects to measure plant species cover and diversity on each site. As specified in section 151 of the ordinance, evaluation criteria may be revised occasionally. -
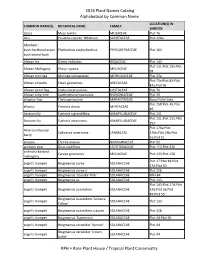
2016 Plant Names Catalog Alphabetical by Common Name
2016 Plant Names Catalog Alphabetical by Common Name LOCATION(S) IN COMMON NAME(S) BOTANICAL NAME FAMILY GARDEN abaca Musa textilis MUSACEAE Plot 76 abiu Pouteria caimito 'Whitman' SAPOTACEAE Plot 128a Abraham- bush:hardhead:scipio- Phyllanthus epiphyllanthus PHYLLANTHACEAE Plot 164 bush:sword-bush African iris Dietes iridioides IRIDACEAE Plot 143 Plot 131:Plot 19a:Plot African Mahogany Khaya nyasica MELIACEAE 58 African moringa Moringa stenopetala MORINGACEAE Plot 32a Plot 71a:Plot 83:Plot African oil palm Elaeis guineensis ARECACEAE 84a:Plot 96 African spiral flag Costus lucanusianus COSTACEAE Plot 76 African tulip-tree Spathodea campanulata BIGNONIACEAE Plot 29 alligator flag Thalia geniculata MARANTACEAE Royal Palm Lake Plot 158:Plot 45:Plot allspice Pimenta dioica MYRTACEAE 46 Amazon lily Eucharis x grandiflora AMARYLLIDACEAE Plot 131 Plot 131:Plot 151:Plot Amazon-lily Eucharis amazonica AMARYLLIDACEAE 152 Plot 176a:Plot American beauty Callicarpa americana LAMIACEAE 176b:Plot 19b:Plot berry 3a:Plot 51 anaqua Ehretia anacua BORAGINACEAE Plot 52 anchovy pear Grias cauliflora LECYTHIDACEAE Plot 112:Plot 32b andiroba:bastard Carapa guianensis MELIACEAE Plot 133:Plot 158 mahogany Plot 17:Plot 18:Plot angel's trumpet Brugmansia aurea SOLANACEAE 27d:Plot 50 angel's trumpet Brugmansia aurea x SOLANACEAE Plot 32b angel's trumpet Brugmansia 'Ecuador Pink' SOLANACEAE RPH-B4 angel's trumpet Brugmansia sp. SOLANACEAE Plot 133 Plot 143:Plot 27d:Plot angel's trumpet Brugmansia suaveolens SOLANACEAE 32b:Plot 3a:Plot 49:Plot 50 Brugmansia suaveolens -

Why Are Plants Carnivorous?
1 ECOPHYSIOLOGICAL LOOK AT PLANT CARNIVORY: Why are plants carnivorous? LUBOMÍR ADAMEC Institute of Botany, Section of Plant Ecology Dukelská 135, CZ-379 82 T řebo ň, Czech Republic 1. Introduction About 650 species of vascular carnivorous (Latin: carnis – flesh, vorare – to swallow) plants occur throughout the world (e.g., Rice, 2006) out of the total of about 300,000 species of vascular plants. Carnivorous plants belong to 15-18 genera of 8-9 botanical families and 5 orders (Givnish, 1989; Juniper et al., 1989; Müller et al., 2004; Heubl et al., 2006; Porembski and Barthlott, 2006; Studni čka, 2006). Due to many remarkable and striking morphological, anatomical, physiological, and ecological features, carnivorous plants have always attracted considerable interest of both researchers and gardeners. Nevertheless, the degree and extent of knowledge of the main disciplines studying this particular ecological functional plant group has always considerably lagged behind the study of non-carnivorous plants. However, similar to the dynamically growing knowledge of non-carnivorous plants, the study of carnivorous plants has developed very rapidly and progressively within the last decade, mainly due to the use of modern molecular taxonomic approaches. Also, modern ecophysiological research of carnivorous plants has progressed considerably within the last decade and has elucidated most of the particulars of carnivorous plants. Thus we are increasingly more able to discuss to what extent carnivorous plants are unique from or common with “normal” non-carnivorous plants. The aim of this paper is to classify and review recent experimental results and concepts concerning plant carnivory from an ecophysiological point of view, with an emphasis on mineral nutrition, growth characteristics, and comparison of aquatic and terrestrial carnivorous plants. -

American Black Nightshade the Wildflower Garden Series a Bog By
Cordia Discovering Florida’s Ethnobotany, Back Cover and Page 12 / Lantana Back Cover and Page 21 ABogby the Highway Unique Flora Faces an Uncertain Future Pages 2 and 6 American Black Nightshade The Wildflower Garden Series by Rufino Osorio Page 9 R. Osorio 2nd Ed., 2003. — Ed.] FNPS Research Endowment Program: a Sample of Projects from 2003-2004 THE FLORIDA NATIVE PLANT SOCIETY has an established Research Endowment Program which every year funds a small number of modest grants to support scientific native plant research. The FNPS This is an older pdf file -- it has formatting Science Advisory Board has worked with a number of excellent grad- uate students, faculty, and other science professionals on these proj- problems when printed with current software. ects. The following are excerpts from proposals funded by the Research Endowment Program during the past two years. 2003 Endowment Awards Demography and Phenology of the Endangered Fern, Ophioglossum palmatum, at the Tosohatchee State Preserve Eliane Norman, Professor emeritus, Stetson University and Sandra Carnival, Field Biologist, Tosohatchee State Preserve and enable researchers to relate phenological patterns in leaf growth, spike production, and maturation to seasonal variation at the Tosohatchee State Preserve. The tasks include: 1. Locate host trees previously identified as bearing Ophioglossum palma- Chapter tum. Note number of ferns per palm and locate side(s) of tree where grows, height at which it grows and any signs of fire. 2. Survey for additional host trees and collect above data. 3. Monitor, on a monthly basis, temperature, relative humidity, and light available at three different locations where the ferns are found. -

Carnivorous Plant Newsletter Vol. 49 No. 3, September 2020
Technical Refereed Contribution Small Butterwort (Pinguicula pumila) in its natural habitat John Bradford • Jensen Beach • Florida • USA George Rogers • Horticulture Department • Palm Beach State College • 3160 PGA Blvd. • Palm Beach Gardens • Florida 33410 • USA • [email protected] Keywords: Pinguicula pumila, flower biology, pollination, stigmatic curling. Abstract: Two wild populations of Small Butterwort (Pinguicula pumila, Lentibulariaceae) were studied during its 2019/2020 late autumn–spring flowering season in Palm Beach County, Florida. The rare yellow-flowered form known primarily from Southwest Florida turned up in East Florida. Except possibly for 19th century literature we were unable to examine, stigmatic curling in response to touch is first reported for Pinguicula. Diverse Dipteran and Hymenopteran floral visitors were observed. Previous indications that spontaneous self-pollination is rare to none in this and related species, especially with reference to cultivated plants, were consistent with our results using insect exclosures on wild plants, whereas open-pollinated flowers made fruits and seeds abundantly. In our area the known populations are all spotty and small, in wet-then-dry disturbed habitats. Introduction South Florida is a great place for native carnivorous plants, with sterile soils, extensive wetlands, and insects aplenty year-round. Here live several species of Drosera, Pinguicula, Utricularia, and, marginally, Sarracenia. The tank bromeliad Catopsis berteroniana hosts bacterial symbionts as di- gestive aids. The present study is a profile of one of the prettiest and most curious local insectivores, Small Butterwort, Pinguicula pumila Michx. Distribution and Habitats Butterworts, comprising the genus Pinguicula, number debatably around 75 species from Can- ada to Chile, and around much of the globe mostly in North Temperate regions (Legendre 2000). -

Carniflora News
THE AUSTRALASIAN CARNIVOROUS PLANTS SOCIETY INC. CARNIFLORA NEWS A.B.N. 65 467 893 226 JULY 2019 Drosera erythrorhiza ssp. squamosa. Photographed by David Colbourn Drosera erythrorhiza ssp. collina. Photographed by Glen Moss Welcome to Carniflora News, a newsletter produced by the Australasian Carnivorous CALENDAR Plants Society Inc. that documents the meetings, news and events of the Society. JULY The current committee of the Australasian Carnivorous Plant Society Inc. comprises: 5th July 2019 - AUSCPS meeting - Canberra featuring bog gardens & winter plant maintenance 12th July 2019 - AUSCPS meeting - Sydney & AGM featuring Winter growing Drosera COMMITTEE AUGUST 2nd August 2019 - AUSCPS meeting - Canberra featuring Cephalotus and Heliamphora President - Wesley Fairhall 9th August 2019 - AUSCPS meeting - Sydney featuring Pinguicula SEPTEMBER 6th September 2019 - AUSCPS meeting - Canberra featuring Pinguicula and Utricularia Vice President - David Colbourn 13th September 2019 - AUSCPS meeting - Sydney featuring Nepenthes [email protected] OCTOBER Treasurer - David Colbourn 5th October 2019 - AUSCPS meeting - Canberra featuring Nepenthes [email protected] 11-13th October 2019 - Southern Orchid Spectacular 12th October 2019 - AUSCPS meeting - Sydney featuring Summer growing Drosera Secretary - Kirk ‘Füzzy’ Hirsch NOVEMBER [email protected] 1st November 2019 - AUSCPS meeting - Canberra featuring Sarracenia 8th November 2019 - AUSCPS meeting - Sydney featuring Sarracenia and Darlingtonia General Committee Member - Barry Bradshaw -

Drosera , Video and Information Night
ISSN 1033-6966 Victorian Ca rnivorous PLANT SOCI ET YINC. Reg No. A0001683Y September 2 015 VCPS Newsletter No. 1 Nepenthes pitopangii Victorian Ca rnivorous PLANT SOCI ET YINC. Newsletter No. 1 September 2015 Office Bearers: July 2015 – June 2016 President Stephen Fretwell – Tel: (03) 8786 8409 email: [email protected] Vice President Sean Spence – Tel: (03) 9743 5809 email: [email protected] General/Member Secretary Peter Bloem – Tel: (03) 9744 2265 email: [email protected] Minutes Secretary Andrew Gibbons email: [email protected] Journal Editor Stephen Fretwell – Tel: (03) 8786 8409 email: [email protected] Assistant Journal Editor David Petch email: [email protected] Internet Co-ordinator Andrew Gibbons email: [email protected] Treasurer Ken Neal – Tel: (03) 9579 4802 Librarian Peter Nisbet – Tel: (03) 9570 5401 Seedbank Administrator Ron Abernethy – Tel: (03) 9879 0908 email: [email protected] Other Publications & Journal distributor Gordon Ohlenrott –Tel: (03) 9878 6596 email: [email protected] Hardware Co-ordinator Andre Cleghorn – Tel: (03) 9584 2087 email: [email protected] Event Co-ordinators Stephen Fretwell – Tel: (03) 8786 8409 email: [email protected] Open Day Liaison Officer Stephen Fretwell – Tel: (03) 8786 8409 email: [email protected] Julian Weston – 0413 041 547 email: julianweston [email protected] Field Trips Organiser Sean Spence – Tel: (03) 9743 5809 email: [email protected] Public Officer Alexander Whitehouse – Tel: (03) 9817 3506 Sales Administrator Ron Abernethy – Tel: (03) 9879 0908 email: [email protected] 2 – VCPS MEETING TOPICS & DATES for 2015 VICTORIAN CARNIVOROUS P LANT SOC IETY This year we have scheduled the following discussion topics, and events: January (31st) New Year BBQ at Ron Abernethy’s House 12.30pm Dionaea muscipula (VFT) .