Carnivorous Plant Responses to Resource Availability
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Status of Insectivorous Plants in Northeast India
Technical Refereed Contribution Status of insectivorous plants in northeast India Praveen Kumar Verma • Shifting Cultivation Division • Rain Forest Research Institute • Sotai Ali • Deovan • Post Box # 136 • Jorhat 785 001 (Assam) • India • [email protected] Jan Schlauer • Zwischenstr. 11 • 60594 Frankfurt/Main • Germany • [email protected] Krishna Kumar Rawat • CSIR-National Botanical Research Institute • Rana Pratap Marg • Lucknow -226 001 (U.P) • India Krishna Giri • Shifting Cultivation Division • Rain Forest Research Institute • Sotai Ali • Deovan • Post Box #136 • Jorhat 785 001 (Assam) • India Keywords: Biogeography, India, diversity, Red List data. Introduction There are approximately 700 identified species of carnivorous plants placed in 15 genera of nine families of dicotyledonous plants (Albert et al. 1992; Ellison & Gotellli 2001; Fleischmann 2012; Rice 2006) (Table 1). In India, a total of five genera of carnivorous plants are reported with 44 species; viz. Utricularia (38 species), Drosera (3), Nepenthes (1), Pinguicula (1), and Aldrovanda (1) (Santapau & Henry 1976; Anonymous 1988; Singh & Sanjappa 2011; Zaman et al. 2011; Kamble et al. 2012). Inter- estingly, northeastern India is the home of all five insectivorous genera, namely Nepenthes (com- monly known as tropical pitcher plant), Drosera (sundew), Utricularia (bladderwort), Aldrovanda (waterwheel plant), and Pinguicula (butterwort) with a total of 21 species. The area also hosts the “ancestral false carnivorous” plant Plumbago zelayanica, often known as murderous plant. Climate Lowland to mid-altitude areas are characterized by subtropical climate (Table 2) with maximum temperatures and maximum precipitation (monsoon) in summer, i.e., May to September (in some places the highest temperatures are reached already in April), and average temperatures usually not dropping below 0°C in winter. -

Reports Ecological Analyses of Relationships Between Essen- and Frost 1991, Ellison and Gotelli 2002)
Ecology, 86(7), 2005, pp. 1737±1743 q 2005 by the Ecological Society of America PREY ADDITION ALTERS NUTRIENT STOICHIOMETRY OF THE CARNIVOROUS PLANT SARRACENIA PURPUREA AMY E. WAKEFIELD,1 NICHOLAS J. GOTELLI,1,3 SARAH E. WITTMAN,1 AND AARON M. ELLISON2 1University of Vermont, Burlington, Vermont 05405 USA 2Harvard University, Harvard Forest, P.O. Box 68, Petersham, Massachusetts 01366 USA Abstract. The carnivorous pitcher plant Sarracenia purpurea receives nutrients from both captured prey and atmospheric deposition, making it a good subject for the study of ecological stoichiometry and nutrient limitation. We added prey in a manipulative ®eld experiment and measured nutrient accumulation in pitcher-plant tissue and pitcher liquid, as well as changes in plant morphology, growth, and photosynthetic rate. Prey addition had no effect on traditional measures of nutrient limitation (leaf morphology, growth, or pho- tosynthetic rate). However, stoichiometric measures of nutrient limitation were affected, as the concentration of both N and P in the leaf tissue increased with the addition of prey. Pitcher ¯uid pH and nitrate concentration did not vary among treatments, although dissolved oxygen levels decreased and ammonia levels increased with prey addition. Ratios of N:P, N:K, and K:P in pitcher-plant tissues suggest that prey additions shifted these carnivorous plants from P limitation under ambient conditions to N limitation with the addition of prey. Key words: carnivorous plants; ®eld experiment; K:P ratio; N:K ratio; N:P ratio; nitrogen; nutrient limitation; phosphorus; Sarracenia purpurea; stoichiometry. INTRODUCTION many species alter production or morphology of car- nivorous organs in response to nutrient input (Knight Reports Ecological analyses of relationships between essen- and Frost 1991, Ellison and Gotelli 2002). -

Quite a Few Reasons for Calling Carnivores 'The Most Wonderful
Annals of Botany 109: 47–64, 2012 doi:10.1093/aob/mcr249, available online at www.aob.oxfordjournals.org REVIEW Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’ Elz˙bieta Kro´l1,*,†, Bartosz J. Płachno2,†, Lubomı´r Adamec3, Maria Stolarz1, Halina Dziubin´ska1 and Kazimierz Tre˛bacz1 1Department of Biophysics, Institute of Biology, Maria Curie-Skłodowska University, Akademicka 19, 20-033 Lublin, Poland, 2Department of Plant Cytology and Embryology, Jagiellonian University, Grodzka 52, 31-044 Cracow, Poland and 3Institute of Botany AS CR, Dukelska´ 135, 37982 Trˇebonˇ, Czech Republic †These authors contributed equally to this work. * For correspondence. E-mail [email protected] Received: 30 May 2011 Returned for revision: 28 June 2011 Accepted: 8 August 2011 Published electronically: 21 September 2011 Downloaded from † Background A plant is considered carnivorous if it receives any noticeable benefit from catching small animals. The morphological and physiological adaptations to carnivorous existence is most complex in plants, thanks to which carnivorous plants have been cited by Darwin as ‘the most wonderful plants in the world’. When considering the range of these adaptations, one realizes that the carnivory is a result of a multitude of different features. † Scope This review discusses a selection of relevant articles, culled from a wide array of research topics on plant carnivory, and focuses in particular on physiological processes associated with active trapping and digestion of http://aob.oxfordjournals.org/ prey. Carnivory offers the plants special advantages in habitats where nutrient supply is scarce. Counterbalancing costs are the investments in synthesis and the maintenance of trapping organs and hydrolysing enzymes. -

Evaluating the Adaptive Evolutionary Convergence of Carnivorous Plant Taxa Through Functional Genomics
Evaluating the adaptive evolutionary convergence of carnivorous plant taxa through functional genomics Gregory L. Wheeler and Bryan C. Carstens Department of Evolution, Ecology, & Organismal Biology, The Ohio State University, Columbus, OH, United States of America ABSTRACT Carnivorous plants are striking examples of evolutionary convergence, displaying complex and often highly similar adaptations despite lack of shared ancestry. Using available carnivorous plant genomes along with non-carnivorous reference taxa, this study examines the convergence of functional overrepresentation of genes previously implicated in plant carnivory. Gene Ontology (GO) coding was used to quantitatively score functional representation in these taxa, in terms of proportion of carnivory- associated functions relative to all functional sequence. Statistical analysis revealed that, in carnivorous plants as a group, only two of the 24 functions tested showed a signal of substantial overrepresentation. However, when the four carnivorous taxa were analyzed individually, 11 functions were found to be significant in at least one taxon. Though carnivorous plants collectively may show overrepresentation in functions from the predicted set, the specific functions that are overrepresented vary substantially from taxon to taxon. While it is possible that some functions serve a similar practical purpose such that one taxon does not need to utilize both to achieve the same result, it appears that there are multiple approaches for the evolution of carnivorous function in plant genomes. Our approach could be applied to tests of functional convergence in other systems provided on the availability of genomes and annotation data for a group. Submitted 27 October 2017 Accepted 13 January 2018 Subjects Bioinformatics, Evolutionary Studies, Genomics, Plant Science Published 31 January 2018 Keywords Carnivorous plants, Gene Ontology, Functional genomics, Convergent evolution Corresponding author Gregory L. -

The Miniature Genome of a Carnivorous Plant Genlisea Aurea
Leushkin et al. BMC Genomics 2013, 14:476 http://www.biomedcentral.com/1471-2164/14/476 RESEARCH ARTICLE Open Access The miniature genome of a carnivorous plant Genlisea aurea contains a low number of genes and short non-coding sequences Evgeny V Leushkin1,2, Roman A Sutormin1, Elena R Nabieva1, Aleksey A Penin1,2,3, Alexey S Kondrashov1,4 and Maria D Logacheva1,5* Abstract Background: Genlisea aurea (Lentibulariaceae) is a carnivorous plant with unusually small genome size - 63.6 Mb – one of the smallest known among higher plants. Data on the genome sizes and the phylogeny of Genlisea suggest that this is a derived state within the genus. Thus, G. aurea is an excellent model organism for studying evolutionary mechanisms of genome contraction. Results: Here we report sequencing and de novo draft assembly of G. aurea genome. The assembly consists of 10,687 contigs of the total length of 43.4 Mb and includes 17,755 complete and partial protein-coding genes. Its comparison with the genome of Mimulus guttatus, another representative of higher core Lamiales clade, reveals striking differences in gene content and length of non-coding regions. Conclusions: Genome contraction was a complex process, which involved gene loss and reduction of lengths of introns and intergenic regions, but not intron loss. The gene loss is more frequent for the genes that belong to multigenic families indicating that genetic redundancy is an important prerequisite for genome size reduction. Keywords: Genome reduction, Carnivorous plant, Intron, Intergenic region Background evolutionary and functional points of view. In a model In spite of the similarity of basic cellular processes in eu- plant species, Arabidopsis thaliana, number of protein- karyotes, their genome sizes are extraordinarily variable. -

Carnivorous Plant Newsletter Vol 48 No 4 December 2019
Drosera in the western USA—an update Barry A. Rice • Center for Plant Diversity • University of California • One Shields Avenue • Davis • California 95616 • USA • [email protected] Keywords: Taxonomy: Drosera, western USA, Drosera anglica, Drosera intermedia, Drosera linearis, Drosera × obovata, Drosera rotundifolia, Drosera × woodii. One of my primary research activities focuses on carnivorous plants in the US states of Montana, Wyoming, Colorado, New Mexico, and westward (but excluding Alaska). Throughout most of this range, the only native species of Drosera are Drosera rotundifolia L., Drosera anglica Huds., and the hybrid Drosera × obovata Mert. & Koch. These species are well-known to readers of this jour- nal, and need no further description. In this region, these plants quite often occur in sites that are widely separated, and in some states these taxa are of considerable rarity (for example, in Colorado D. rotundifolia and D. anglica are known at only 4 and 1 sites, respectively; Wolf et al. 2006). In addition to these species, there have been reports of two additional native Drosera—Drosera intermedia Hayne in northern Idaho (and perhaps adjacent eastern Washington), and Drosera lin- earis Goldie in Montana. Drosera intermedia was reported in Boundary County, Idaho by Bursik (1993), as growing with Drosera anglica, but being quite different in form. Additional populations were subsequently found in Custer County, Idaho (L. Kinter, pers. comm. 2017). Of course, the closest other Drosera inter- media plants were on the other side of the continent, so this was considered a find of great interest, and the plants were treated as of special significance. A few years ago, I decided I needed to visit and assess these plants. -

Florida Council of Bromeliad Societies, Inc
Florida Council of Bromeliad Societies, Inc. In This Issue: 2007 Shows and Sales Cold Hardy Bromeliads List Vol. 27 Issue 1 February 2007 FCBS Affiliated Societies and Representatives B. Guild Tampa Bay Caloosahatchee Tom Wolfe Vicky Chirnside 5211 Lake LeClare Road 951 Southland Road Lutz 33558 Venice 34293 813-961-1475 941-493-5825 [email protected] [email protected] Bob Teems Tom Foley 813-855-0938 239-458-4656 Broward County Fl. East Coast Jose Donayre Calandra Thurrott 1240 Jefferson St. 713 Breckenridge Drive Hollywood 33019-1807 Port Orange 32127 954-925-5112 386-761-4804 Jcadonayre @bellsouth.net [email protected] Colleen Hendrix Carolyn Schoenau 954-530-0076 352-372-6589 Central Florida F. West Coast Betsy McCrory Linda Sheetz 3615 Boggy Creek Rd. 1153 Williams Dr. S Kissimmee 34744 St. Petersburg 33705 407-348-2139 727-864-3165 [email protected] [email protected] Butch Force Brian Corey 407-886-4814 727-864-3165 South Florida Gainesville Juan Espinosa-Almodovar Al Muzzell P.O. Box 430722 P.O. Box 14442 Miami 33243 Gainesville 32604 305-667-6155 352-372-4576 [email protected] John R. Moxley Michael Michalski 352-528-0783 305-279-2416 (Continued on the inside back cover.) 2007 Bromeliad Extravaganza Presented by Florida Council of Bromeliad Societies Hosted by the Bromeliad Society of Broward County Saturday, September 29, 2007 at the Hilton Ft. Lauderdale Airport Hotel 1870 Griffin Rd. Dania Beach, FL 33004 954-920-3300 954-920-3348 (fax) Room rates: Single or double $89.00 Rates in effect until September 14, 2007 Sale, Banquet, Raffle and Rare Plant Auction will take place at the same location. -

A New Carnivorous Plant Lineage (Triantha) with a Unique Sticky-Inflorescence Trap
A new carnivorous plant lineage (Triantha) with a unique sticky-inflorescence trap Qianshi Lina,b,1, Cécile Anéc,d, Thomas J. Givnishc, and Sean W. Grahama,b aDepartment of Botany, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; bUBC Botanical Garden, University of British Columbia, Vancouver, BC V6T 1Z4, Canada; cDepartment of Botany, University of Wisconsin–Madison, Madison, WI 53706; and dDepartment of Statistics, University of Wisconsin–Madison, Madison WI 53706 Edited by Elizabeth A. Kellogg, Donald Danforth Plant Science Center, St. Louis, MO, and approved June 5, 2021 (received for review October 30, 2020) Carnivorous plants consume animals for mineral nutrients that and in wetlands, including bogs, marly shorelines, and calcareous enhance growth and reproduction in nutrient-poor environments. spring-fed fens. In bogs, T. occidentalis is commonly found with Here, we report that Triantha occidentalis (Tofieldiaceae) represents recognized carnivorous plants such as Drosera rotundifolia a previously overlooked carnivorous lineage that captures insects on (Droseraceae) and Pinguicula vulgaris (Lentibulariaceae). During sticky inflorescences. Field experiments, isotopic data, and mixing the summer flowering season, T. occidentalis produces leafless models demonstrate significant N transfer from prey to Triantha, erect flowering stems up to 80 cm tall (12). These scapes have with an estimated 64% of leaf N obtained from prey capture in sticky glandular hairs, especially on their upper portions, a feature previous years, comparable to levels inferred for the cooccurring distinguishing Triantha from other genera of Tofieldiaceae round-leaved sundew, a recognized carnivore. N obtained via carnivory (Fig. 1). Small insects are often found trapped by these hairs; is exported from the inflorescence and developing fruits and may herbarium specimens are frequently covered in insects (Fig. -

The Microbial Phyllogeography of the Carnivorous Plant Sarracenia Alata
Microb Ecol (2011) 61:750–758 DOI 10.1007/s00248-011-9832-9 PLANT MICROBE INTERACTIONS The Microbial Phyllogeography of the Carnivorous Plant Sarracenia alata Margaret M. Koopman & Bryan C. Carstens Received: 6 November 2010 /Accepted: 15 February 2011 /Published online: 24 March 2011 # Springer Science+Business Media, LLC 2011 Abstract Carnivorous pitcher plants host diverse microbial Introduction communities. This plant–microbe association provides a unique opportunity to investigate the evolutionary process- The integration of ecosystem genetics, phylogenetics, and es that influence the spatial diversity of microbial commu- community ecology has provided important insights into nities. Using next-generation sequencing of environmental the diversity, assembly, evolution, and functionality of samples, we surveyed microbial communities from 29 communities [1–5]. By exploring ecosystems in an evolu- pitcher plants (Sarracenia alata) and compare community tionary framework, investigators can measure genetic composition with plant genetic diversity in order to interactions across variable temporal and spatial scales explore the influence of historical processes on the and gain insight into fundamental processes such as food population structure of each lineage. Analyses reveal web dynamics and nutrient cycling [1, 3, 4]. Studies that there is a core S. alata microbiome, and that it is integrating these fields initially focused on the genetics of similar in composition to animal gut microfaunas. The plant species that supply a variety of important resources spatial structure of community composition in S. alata and environmental structure to other organisms in the (phyllogeography) is congruent at the deepest level with ecosystem [6]. An intriguing extension of these studies, the dominant features of the landscape, including the and an important opportunity for community geneticists, is Mississippi river and the discrete habitat boundaries that to further investigate community level responses to host– the plants occupy. -
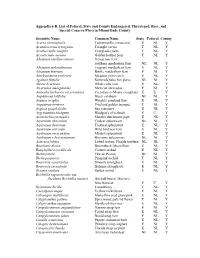
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2013 South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae) Lendel, Anita Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-93287 Dissertation Published Version Originally published at: Lendel, Anita. South American Cacti in time and space: studies on the diversification of the tribe Cereeae, with particular focus on subtribe Trichocereinae (Cactaceae). 2013, University of Zurich, Faculty of Science. South American Cacti in Time and Space: Studies on the Diversification of the Tribe Cereeae, with Particular Focus on Subtribe Trichocereinae (Cactaceae) _________________________________________________________________________________ Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr.sc.nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich von Anita Lendel aus Kroatien Promotionskomitee: Prof. Dr. H. Peter Linder (Vorsitz) PD. Dr. Reto Nyffeler Prof. Dr. Elena Conti Zürich, 2013 Table of Contents Acknowledgments 1 Introduction 3 Chapter 1. Phylogenetics and taxonomy of the tribe Cereeae s.l., with particular focus 15 on the subtribe Trichocereinae (Cactaceae – Cactoideae) Chapter 2. Floral evolution in the South American tribe Cereeae s.l. (Cactaceae: 53 Cactoideae): Pollination syndromes in a comparative phylogenetic context Chapter 3. Contemporaneous and recent radiations of the world’s major succulent 86 plant lineages Chapter 4. Tackling the molecular dating paradox: underestimated pitfalls and best 121 strategies when fossils are scarce Outlook and Future Research 207 Curriculum Vitae 209 Summary 211 Zusammenfassung 213 Acknowledgments I really believe that no one can go through the process of doing a PhD and come out without being changed at a very profound level. -

Carnivorous Plant Newsletter V44 N4 December 2015
Technical Refereed Contribution Photoperiod regulates Cape Sundew (Drosera capensis) gland secretion and leaf development Wang Dong-Hui • College of Life Science • Peking University • Haidian • Beijing 100871 • PRC Wang Dong-Qi • Cui Yi-Wei • Yang Lu • Gu Xiao-Di • Song Wen-Fei • Li Feng • The High School Affiliated to Renmin University of China • Haidian • Beijing 100080 • PRC • lifeng2004@pku. edu.cn Keywords: carnivorous plant, photoperiod, plant development, Drosera capensis. Abstract: Cape Sundew (Drosera capensis), a carnivorous plant that catches flies with sticky mu- cus, has attracted great interest among botanists and horticulture hobbyists since the Darwin era. But little is known about how this carnivorous plant regulates morphogenesis and organ formation to accommodate environmental changes. In this article we present the relationship between gland secretion of Cape Sundew and photoperiod utilizing various physiological and morphological meth- ods. We show that Cape Sundew grows faster and secretes more mucus under long days than under short days. Under long days leaf length and the blade\petiole ratio increases, leading to increased fly catching capacities. More importantly, in the short term, the rhythm of photoperiod causes Cape Sundew to secrete mucus independent of photo intensity. Introduction As one of the most special plant groups, carnivorous plants perform photosynthesis and feed on insects and some large carnivorous plants even prey on birds and small mammals. Darwin believed that a carnivorous plant was one of the most astonishing phenomena in the world (Dar- win 1875; Ellison & Gotelli 2009). Carnivorous plants are represented by more than 600 species belonging to 20 genera (Ellison & Gotelli 2001; McPherson 2010).