Patterns of Colonization and Species Distribution for Azorean Arthropods: Evolution, Diversity, Rarity and Extinction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cally Plant List a ACIPHYLLA Horrida
Cally Plant List A ACIPHYLLA horrida ACONITUM albo-violaceum albiflorum ABELIOPHYLLUM distichum ACONITUM cultivar ABUTILON vitifolium ‘Album’ ACONITUM pubiceps ‘Blue Form’ ACAENA magellanica ACONITUM pubiceps ‘White Form’ ACAENA species ACONITUM ‘Spark’s Variety’ ACAENA microphylla ‘Kupferteppich’ ACONITUM cammarum ‘Bicolor’ ACANTHUS mollis Latifolius ACONITUM cammarum ‘Franz Marc’ ACANTHUS spinosus Spinosissimus ACONITUM lycoctonum vulparia ACANTHUS ‘Summer Beauty’ ACONITUM variegatum ACANTHUS dioscoridis perringii ACONITUM alboviolaceum ACANTHUS dioscoridis ACONITUM lycoctonum neapolitanum ACANTHUS spinosus ACONITUM paniculatum ACANTHUS hungaricus ACONITUM species ex. China (Ron 291) ACANTHUS mollis ‘Long Spike’ ACONITUM japonicum ACANTHUS mollis free-flowering ACONITUM species Ex. Japan ACANTHUS mollis ‘Turkish Form’ ACONITUM episcopale ACANTHUS mollis ‘Hollard’s Gold’ ACONITUM ex. Russia ACANTHUS syriacus ACONITUM carmichaelii ‘Spätlese’ ACER japonicum ‘Aconitifolium’ ACONITUM yezoense ACER palmatum ‘Filigree’ ACONITUM carmichaelii ‘Barker’s Variety’ ACHILLEA grandifolia ACONITUM ‘Newry Blue’ ACHILLEA ptarmica ‘Perry’s White’ ACONITUM napellus ‘Bergfürst’ ACHILLEA clypeolata ACONITUM unciniatum ACIPHYLLA monroi ACONITUM napellus ‘Blue Valley’ ACIPHYLLA squarrosa ACONITUM lycoctonum ‘Russian Yellow’ ACIPHYLLA subflabellata ACONITUM japonicum subcuneatum ACONITUM meta-japonicum ADENOPHORA aurita ACONITUM napellus ‘Carneum’ ADIANTUM aleuticum ‘Japonicum’ ACONITUM arcuatum B&SWJ 774 ADIANTUM aleuticum ‘Miss Sharples’ ACORUS calamus ‘Argenteostriatus’ -

Systematic Studies of the South African Campanulaceae Sensu Stricto with an Emphasis on Generic Delimitations
Town The copyright of this thesis rests with the University of Cape Town. No quotation from it or information derivedCape from it is to be published without full acknowledgement of theof source. The thesis is to be used for private study or non-commercial research purposes only. University Systematic studies of the South African Campanulaceae sensu stricto with an emphasis on generic delimitations Christopher Nelson Cupido Thesis presented for the degree of DOCTOR OF PHILOSOPHY in the Department of Botany Town UNIVERSITY OF CAPECape TOWN of September 2009 University Roella incurva Merciera eckloniana Microcodon glomeratus Prismatocarpus diffusus Town Wahlenbergia rubioides Cape of Wahlenbergia paniculata (blue), W. annularis (white) Siphocodon spartioides University Rhigiophyllum squarrosum Wahlenbergia procumbens Representatives of Campanulaceae diversity in South Africa ii Town Dedicated to Ursula, Denroy, Danielle and my parents Cape of University iii Town DECLARATION Cape I confirm that this is my ownof work and the use of all material from other sources has been properly and fully acknowledged. University Christopher N Cupido Cape Town, September 2009 iv Systematic studies of the South African Campanulaceae sensu stricto with an emphasis on generic delimitations Christopher Nelson Cupido September 2009 ABSTRACT The South African Campanulaceae sensu stricto, comprising 10 genera, represent the most diverse lineage of the family in the southern hemisphere. In this study two phylogenies are reconstructed using parsimony and Bayesian methods. A family-level phylogeny was estimated to test the monophyly and time of divergence of the South African lineage. This analysis, based on a published ITS phylogeny and an additional ten South African taxa, showed a strongly supported South African clade sister to the campanuloids. -

Campanulaceae) Based on ITS and Tranl-F Sequence Data: Implications for a Reclassification
CORE Metadata, citation and similar papers at core.ac.uk Provided by University of the Western Cape Research Repository Cupido, C. N. et al. (2013). Phylogeny of Southern African and Australasian Wahlenbergioids (Campanulaceae) based on ITS and tranL-F sequence data: implications for a reclassification. Systematic Botany, 38(2): 523 – 535 http:// doi.org/10.1600/036364413X666714 dx. Phylogeny of Southern African and Australasian Wahlenbergioids (Campanulaceae) based on ITS and trnL-F sequence data: implications for a reclassification Christopher N. Cupido , Jessica M. Prebble , and William M. M. Eddie Abstract The Campanulaceae: Wahlenbergioideae currently comprises 15 genera, one of which, Wahlenbergia, is widespread over the southern continents. Southern Africa is the region with maximum wahlenbergioid diversity with 12 genera and approximately 252 species. A second center is Australasia with 38 Wahlenbergia species. This study used a broad sample of wahlenbergioid diversity from South Africa, Australia, and New Zealand to reconstruct a phylogeny based on chloroplast trnL-F and nuclear ITS sequences. Data were analyzed separately and in combination using parsimony and Bayesian methods. The results suggest that for the wahlenbergioids to be monophyletic Wahlenbergia hederacea has to be excluded and that none of the South African, Australian or New Zealand lineages are strictly monophyletic. There are five species assemblages that are in some disagreement with current classification in the family. Wahlenbergia, Prismatocarpus and Roella are shown to be non-monophyletic and implications for a reclassification are presented. Careful consideration of morphological characters is suggested before the adjustment of generic circumscriptions can be accomplished. Recent family-wide molecular phylogenetic studies have supported the view that the Campanulaceae s.s. -

Informação Base De Biodiversidade Da Ilha Do Corvo E Do Ilhéu De Vila Franca Do Campo
LIFE+ Safe Islands for Seabirds Relatório Acção A1 - Informação Base de Biodiversidade da Ilha do Corvo e do Ilhéu de Vila Franca do Campo LIFE07 NAT/P/000649 Corvo, Dezembro 2009 O P r o j e c O O projecto LIFE+ Safe Islands for Seabirds é uma parceria da SPEA com a Secretaria Regional do Ambiente e do Mar (SRAM), a Câmara Municipal do Corvo e a Royal Society for Protection of Birds, contando ainda com o apoio das seguintes entidades enquanto observadoras na sua Comissão Executiva: Direcção Regional dos Recursos Florestais (DRRF) e Câmara Municipal de Vila Franca do Campo. Trabalhar para o estudo e conservação das aves e seus habitats, promovendo um desenvolvimento que garanta a viabilidade do património natural para usufruto das gerações futuras. A SPEA – Sociedade Portuguesa para o Estudo das Aves é uma organização não governamental de ambiente que trabalha para a conservação das aves e dos seus habitats em Portugal. Como associação sem fins lucrativos, depende do apoio dos sócios e de diversas entidades para concretizar as suas acções. Faz parte de uma rede mundial de organizações de ambiente, a BirdLife International, que actua em mais de 100 países e tem como objectivo a preservação da diversidade biológica através da conservação das aves, dos seus habitats e da promoção do uso sustentável dos recursos naturais. LIFE+ Safe Islands for Seabirds. Relatório Inicial Sociedade Portuguesa para o Estudo das Aves, 2009 Direcção Nacional: Ricardo Azul Tomé, Maria Ana Peixe, Pedro Guerreiro, Ana Leal Martins, João Jara, Paulo Travassos, Pedro Coelho, Miguel Capelo, Paulo Simões Coelho, Teresa Catry Direcção Executiva: Luís Costa Coordenação do projecto: Pedro Luís Geraldes Equipa técnica: Ana Catarina Henriques, Carlos Silva, Joana Domingues, Nuno Oliveira, Sandra Hervías, Nuno Domingos, Susana Costa e Vanessa Oliveira. -

The Occurrence of Red and Yellow Autumn Leaves Explained by Regional Differences in Insolation and Temperature
Review Tansley review The occurrence of red and yellow autumn leaves explained by regional differences in insolation and temperature Authors for correspondence: Susanne S. Renner1 and Constantin M. Zohner2 Susanne S. Renner 1 2 Tel: +49 89 17861 250 Systematic Botany and Mycology, University of Munich (LMU), Menzinger Str. 67, Munich 80638, Germany ; Institute of Email: [email protected] Integrative Biology, Department of Environmental Systems Science, ETH Zurich, Zurich 8092, Switzerland Constantin M. Zohner Email: [email protected] Received: 19 January 2019 Accepted: 24 April 2019 Contents Summary 1464 IV. The adaptive value of colour-changing leaves 1468 I. Introduction 1464 V. Outlook 1469 II. Phylogenetic and geographical occurrence of autumn colour Acknowledgements 1469 change 1465 References 1470 III. Physiological functions of autumnal leaf xanthophylls and anthocyanins 1466 Summary New Phytologist (2019) 224: 1464–1471 Red or yellow autumn leaves have long fascinated biologists, but their geographical doi: 10.1111/nph.15900 concentration in trees in Eastern North America (ENA) has defied evolutionary explanations. In this review, anthocyanins and xanthophylls are discussed in relation to their occurrence in Key words: adaptive explanation, different regions of the Northern Hemisphere, phylogenetic distribution and photoprotective anthocyanins, photo-oxidative damage, function during the breakdown of chlorophylls. Pigments in senescing leaves that intercept regional climates, solar irradiation, incident light and dissipate the absorbed energy extend the time available for nutrient resorption. xanthophylls. Experiments with Arabidopsis have revealed greatest anthocyanin photoprotective function at low temperatures and high light intensities, and high-resolution solar irradiation maps reveal that ENA and Asia receive higher irradiation than does Europe. -
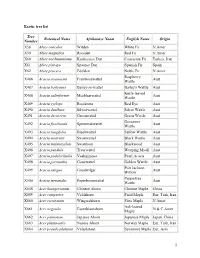
1 Exotic Tree List Tree Number Botanical Name Afrikaanse Naam
Exotic tree list Tree Botanical Name Afrikaanse Naam English Name Origin Number X58 Abies concolor Witden White Fir N.Amer X59 Abies magnifica Rooiden Red Fir N.Amer X60 Abies nordmanniana Kaukasiese Den Caucasian Fir Turkey, Iran X61 Abies pinsapo Spaanse Den Spanish Fir Spain X62 Abies procera Edelden Noble Fir N.Amer Raspberry X486 Acacia acuminata Frambosewattel Aust Wattle X487 Acacia baileyana Bailey-se-wattel Bailey's Wattle Aust Knife-leaved X488 Acacia cultriformis Mesblaarwattel Aust Wattle X489 Acacia cyclops Rooikrans Red Eye Aust X490 Acacia dealbata Silwerwattel Silver Wattle Aust X491 Acacia decurrens Groenwattel Green Wattle Aust Gossamer X492 Acacia floribunda Spinnerakwattel Aust Wattle X493 Acacia longifolia Bleekwattel Sallow Wattle Aust X494 Acacia mearnsii Swartwattel Black Wattle Aust X495 Acacia melanoxylon Swarthout Blackwood Aust X496 Acacia pendula Treurwattel Weeping Myall Aust X497 Acacia podalyriifolia Vaalmimosa Pearl Acacia Aust X498 Acacia pycnantha Gouewattel Golden Wattle Aust Port Jackson X499 Acacia saligna Goudwilger Aust Willow Peppertree X500 Acacia terminalis Peperboomwattel Aust Wattle X658 Acer buergerianum Chinese Ahorn Chinese Maple China X659 Acer campestre Veldahorn Field Maple Eur, Turk, Iran X660 Acer circinatum Wingerdahorn Vine Maple N Amer Ash-leaved X661 Acer negundo Essenblaarahorn N & C Amer Maple X662 Acer palmatum Japanse Ahorn Japanese Maple Japan, China X663 Acer platanoides Noorse Ahorn Norway Maple Eur, Turk, Iran X664 Acer pseudo-platanus Valsplataan Sycamore Maple Eur, Asia -

Phylogeny and Biogeography of Isophyllous Species of Campanula (Campanulaceae) in the Mediterranean Area
Systematic Botany (2006), 31(4): pp. 862–880 # Copyright 2006 by the American Society of Plant Taxonomists Phylogeny and Biogeography of Isophyllous Species of Campanula (Campanulaceae) in the Mediterranean Area JEONG-MI PARK,1 SANJA KOVACˇ IC´ ,2 ZLATKO LIBER,2 WILLIAM M. M. EDDIE,3 and GERALD M. SCHNEEWEISS4,5 1Department of Systematic and Evolutionary Botany, Institute of Botany, University of Vienna, Rennweg 14, A-1030 Vienna, Austria; 2Botanical Department and Botanical Garden of the Faculty of Science, University of Zagreb, HR-10000 Zagreb, Croatia; 3Office of Lifelong Learning, University of Edinburgh, 11 Buccleuch Place, Edinburgh, EH8 9LW, Scotland, U.K.; 4Department of Biogeography and Botanical Garden, Institute of Botany, University of Vienna, Rennweg 14, A-1030 Vienna, Austria 5Author for correspondence ([email protected]) Communicating Editor: Thomas G. Lammers ABSTRACT. Sequence data from the nuclear internal transcribed spacer (ITS) were used to infer phylogenetic relationships within a morphologically, karyologically, and geographically well-defined group of species of Campanula (Campanulaceae), the Isophylla group. Although belonging to the same clade within the highly paraphyletic Campanula, the Rapunculus clade, members of the Isophylla group do not form a monophyletic group but fall into three separate clades: (i) C. elatines and C. elatinoides in the Alps; (ii) C. fragilis s.l. and C. isophylla with an amphi-Tyrrhenian distribution; and (iii) the garganica clade with an amphi-Adriatic distribution, comprised of C. fenestrellata s.l., C. garganica s.l., C. portenschlagiana, C. poscharskyana, and C. reatina. Taxa currently classified as subspecies of C. garganica (garganica, cephallenica, acarnanica) and C. fenestrellata subsp. -

Ecological Basis for the Control of Gunnera Tinctoria in Sao Miguel
Second International Weed Control Congress Copenhagen 1996 . Ecological basis for the control of Gunnera tinctoria in Sao Miguel . Island By L sn.. v A, JT A V ARES and A PENA Departamento de Biologia, Universidade dos A(:ores, PT-9500 Ponta Dell?lUia, Portugal, E-mail [email protected] Summary Gunnera tinctoria, an herbaceous plant from South America, is naturalised in Sao Miguel island (Azores) . .In this research an ecologically based strategy for G. tinctoria control is suggested. Infestation structure, altitudinal range, associated plants, phenology and natural enemies were studied. G. tillctoria was found from \00 to 900 m of altitude, in plane or highly sloped terrain, on rich soil or gravel, in roadsides, trails, and water streams. Infestation foci were found at 40 Krn from introduction site. Populations consisted of isolated or small groups of plants, with reduced cover, associated with other weeds. According to three plant invaders classification systems this plant presents several negative characters in terms of conservation: high seed production, vegetative reproduction, high impact on the landscape. invasion of natural vegetation. Priority of control should be given to satellite populations in high conservation ·value sites. Control of heavier infestations will need a persistent and global approach. Introduction Natural populations of Gunnera sp. are restricted to super-humid areas with heavy rainfall; they prefer high altitudes and open or lightly shaded areas, and are often pioneers on bare land (Bergman et al., 1992). The Gunneracea includes the genus Gunnera L., with terrestrial, rhizomatous, perennial herbs, sometimes gigantic, from tropical and warm temperate regions. The larger kinds are grown for the striking effect of their enormous leaves, the smaller forms. -

A Common Threat to IUCN Red-Listed Vascular Plants in Europe
Tourism and recreation: a common threat to IUCN red-listed vascular plants in Europe Author Ballantyne, Mark, Pickering, Catherine Marina Published 2013 Journal Title Biodiversity and Conservation DOI https://doi.org/10.1007/s10531-013-0569-2 Copyright Statement © 2013 Springer. This is an electronic version of an article published in Biodiversity and Conservation, December 2013, Volume 22, Issue 13-14, pp 3027-3044. Biodiversity and Conservation is available online at: http://link.springer.com/ with the open URL of your article. Downloaded from http://hdl.handle.net/10072/55792 Griffith Research Online https://research-repository.griffith.edu.au Manuscript 1 Tourism and recreation: a common threat to IUCN red-listed vascular 1 2 3 4 2 plants in Europe 5 6 7 8 3 *Mark Ballantyne and Catherine Marina Pickering 9 10 11 12 4 Environmental Futures Centre, School of Environment, Griffith University, Gold Coast, 13 14 5 Queensland 4222, Australia 15 16 17 18 6 *Corresponding author email: [email protected], telephone: +61(0)405783604 19 20 21 7 22 23 8 24 25 9 26 27 28 10 29 30 11 31 32 12 33 34 13 35 36 37 14 38 39 15 40 41 16 42 43 17 44 45 46 18 47 48 19 49 50 20 51 52 21 53 54 55 22 56 57 23 58 59 24 60 61 62 63 64 65 25 Abstract 1 2 3 4 26 Tourism and recreation are large industries employing millions of people and contribute over 5 6 27 US$2.01 trillion to the global economy. -

Sommaire Connaissez-Vous Les Aphrodes Du Québec?
SOMMAIRE CONNAISSEZ-VOUS Connaissez-vous les Aphrodes du Québec? 1 LES APHRODES Faisons la lumière sur les espèces de lucioles présentes au Québec _________________ 5 DU QUÉBEC? Un nouvel outil pour les fourmis _________ 9 urtis (1829) a introduit le taxon Aphrodes pour Des insectes gigantesques _____________ 10 Cregrouper sept espèces de Cicadellides de Grande- Aventures avec le Monarque ____________ 12 Bretagne. Ce genre très répandu dans la région La boîte à outils paléarctique a été introduit en Amérique du Nord, vers la fin du 19e siècle (Hamilton 1983). Ultérieurement, Field guide to the flower flies of Northeas- Aphrodes bicincta (Schrank 1776) et A. costata (Panzer tern North America ________________ 14 1799) furent récoltés au Québec, à Coaticook (1913) Insectes. Un monde secret __________ 15 et à Kazabazua (1928) (Site CNCI, consulté 2019). Cerambycidae (Coleoptera) of Canada Les espèces du genre Aphrodes sont difficiles à sépa- and Alaska _______________________ 16 rer avec les caractères morphologiques externes et internes. Le Russe Tishechkin (1998) découvrit que Entomographies _____________________ 17 le groupe A. bicincta représentait trois espèces dif- Cocchenille du genre Acanthococcus _____ 20 férentes, après étude des sonogrammes enregistrés dans la communication des individus émettant des Nouvelles de l'organisme ______________ 20 vibrations transférées au substrat (tige, feuille). En Deux membres fondateurs en deuil _______ 20 Angleterre, des études du gène mitochondrial de la sous-unité I du cytochrome c-oxydase (COI) ont Statistiques des visites au site des fourmis _ 21 consolidé les observations découlant des sonogrammes Rapport des activités de 2018-2019 ______ 22 obtenus avant le sacrifice des spécimens (Bluemelet al. -

GREEN GARDENS AZORES PROJECT: AZORES ABRIEF GARDENS GREEN in the AZORES’ HISTORICAL GARDENS HISTORICAL AZORES’ the in : Azores, Flora De Jardín, Turismo De Jardines
GREEN GARDENS AZORES PROJECT: A BRIEF CHARACTERIZATION OF THE VASCULAR FLORA IN THE AZORES’ HISTORICAL GARDENS Maria João Pereira*, Raimundo Quintal**, Carina Costa*** & Isabel Albergaria**** Abstract The Green Gardens Azores Project is part of the action plan for tourism development in Portugal (2014-2020) aiming to integrate the Azorean Gardens in the circuit of international ‘Garden Tourism’. With that purpose we built a checklist of the vascular plants cultivated in 8 Azorean Historical Gardens. The analysis of this checklist reveals a richness of 1884 specific and infra-specific taxa, hybrids and cultivars. This richness is represented by 168 families, 514 genera, 991 species, 288 hybrids and 958 cultivars. Camellia hybrids correspond to 60% of all the hybrids and Camellia cultivars represent 71% of all the cultivars. Zamiaceae is the family best represented with 73 species while the best represented genera are Encephalartos with 48 species and Camellia with 45 species. The presence of 5 species extinct in the wild and 96 threatened species in the Azorean Gardens stresses the role of the Gardens in the Conservation of World Flora. Keywords: Azores, Garden flora, Garden tourism. EL PROYECTO «JARDINES VERDES DE AZORES»: BREVE CARACTERIZACIÓN DE LA FLORA VASCULAR DE LOS JARDINES HISTÓRICOS DE AZORES 11 Resumen El proyecto «jardines verdes de Azores» es parte de un plan de acción para el desarrollo turístico de Portugal (2014-2020) pretendiendo integrar estos jardines en el circuito inter- nacional de jardines turísticos. Con este propósito hicimos la redacción de un listado de plantas vasculares cultivadas en ocho jardines históricos de Azores. El análisis del listado señaló una riqueza de 1884 categorías específicas, infraespecíficas, híbridos y cultivares. -

Laurisilva of Madeira Portugal
LAURISILVA OF MADEIRA PORTUGAL The Laurisilva of Madeira is the largest surviving relict of a virtually extinct laurel forest type once widespread in Europe. It is still 90% primary forest and is a centre of plant diversity, containing a unique suite of rare and relict plants and animals, especially endemic bryophytes, ferns, vascular plants, animals such as the Madeiran long-toed pigeon and a very rich invertebrate fauna. COUNTRY Portugal NAME Laurisilva of Madeira NATURAL WORLD HERITAGE SITE 1999: Inscribed on the World Heritage List under Natural Criteria ix and x. STATEMENT OF OUTSTANDING UNIVERSAL VALUE The UNESCO World Heritage Committee adopted the following Statement of Outstanding Universal Value at the time of inscription: Brief Synthesis The Laurisilva of Madeira, within the Parque Natural da Madeira (Madeira Natural Park) conserves the largest surviving area of primary laurel forest or "laurisilva", a vegetation type that is now confined to the Azores, Madeira and the Canary Islands. These forests display a wealth of ecological niches, intact ecosystem processes, and play a predominant role in maintaining the hydrological balance on the Island of Madeira. The property has great importance for biodiversity conservation with at least 76 vascular plant species endemic to Madeira occurring in the property, together with a high number of endemic invertebrates and two endemic birds including the emblematic Madeiran Laurel Pigeon. Criterion (ix): The Laurisilva of Madeira is an outstanding relict of a previously widespread laurel forest type, which covered much of Southern Europe 15-40 million years ago. The forest of the property completely covers a series of very steep, V-shaped valleys leading from the plateau and east-west ridge in the centre of the island to the north coast.