Linking Dendrometry and Dendrochronology in the Dominant Azorean Tree Laurus Azorica (Seub.) Franco
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Picconia Excelsa (Oleaceae)
situation on scoria (e.g. Rangitoto) and perhaps there for the same reasons (excellent drainage? aeration of roots?). Other matters of interest and/or concern were the site of the old native plant plantation and the tragic devastation wrought by possums on the pohutukawas. All our thanks to the leader and the organisers. Received 5 July 1989. PICCONIA EXCELSA (OLEACEAE) R.O. Gardner The current Auckland City Council Proposed District Scheme lists two Picconia excelsa trees in Remuera at St Kentigern School and at 115 Victoria Avenue that are to be protected for their botanical (rarity) value. This species the Canary Islands olive is not mentioned in most horticultural works. It forms a tree of medium size heavy in foliage with the rough bark and crooked branching typical of its family. Sterile specimens have a deceptive likeness to those of Nestegis spp. even having a waxy bloom on the petioles — (like N. cunninghamii but not N. apetala) (Johnson 1957). The older AK material was first determined by Dr Peter Green of Kew. There are several other individuals in Auckland like the above of good size but almost certainly less than a hundred years old. There are trees at 2 Woodside Ave Mt Eden at 277 Mt Eden Rd and on a farmhouse drive in Ihumatao Rd; there is a AK specimen from the Wilson Home in Takapuna and until recently there was a tree in the crater of Mt St John. Perhaps the importation of Picconia might be the result of a nurserymans recognizing that Canary Islands species should flourish in what is literally the opposite side of the world. -

Cally Plant List a ACIPHYLLA Horrida
Cally Plant List A ACIPHYLLA horrida ACONITUM albo-violaceum albiflorum ABELIOPHYLLUM distichum ACONITUM cultivar ABUTILON vitifolium ‘Album’ ACONITUM pubiceps ‘Blue Form’ ACAENA magellanica ACONITUM pubiceps ‘White Form’ ACAENA species ACONITUM ‘Spark’s Variety’ ACAENA microphylla ‘Kupferteppich’ ACONITUM cammarum ‘Bicolor’ ACANTHUS mollis Latifolius ACONITUM cammarum ‘Franz Marc’ ACANTHUS spinosus Spinosissimus ACONITUM lycoctonum vulparia ACANTHUS ‘Summer Beauty’ ACONITUM variegatum ACANTHUS dioscoridis perringii ACONITUM alboviolaceum ACANTHUS dioscoridis ACONITUM lycoctonum neapolitanum ACANTHUS spinosus ACONITUM paniculatum ACANTHUS hungaricus ACONITUM species ex. China (Ron 291) ACANTHUS mollis ‘Long Spike’ ACONITUM japonicum ACANTHUS mollis free-flowering ACONITUM species Ex. Japan ACANTHUS mollis ‘Turkish Form’ ACONITUM episcopale ACANTHUS mollis ‘Hollard’s Gold’ ACONITUM ex. Russia ACANTHUS syriacus ACONITUM carmichaelii ‘Spätlese’ ACER japonicum ‘Aconitifolium’ ACONITUM yezoense ACER palmatum ‘Filigree’ ACONITUM carmichaelii ‘Barker’s Variety’ ACHILLEA grandifolia ACONITUM ‘Newry Blue’ ACHILLEA ptarmica ‘Perry’s White’ ACONITUM napellus ‘Bergfürst’ ACHILLEA clypeolata ACONITUM unciniatum ACIPHYLLA monroi ACONITUM napellus ‘Blue Valley’ ACIPHYLLA squarrosa ACONITUM lycoctonum ‘Russian Yellow’ ACIPHYLLA subflabellata ACONITUM japonicum subcuneatum ACONITUM meta-japonicum ADENOPHORA aurita ACONITUM napellus ‘Carneum’ ADIANTUM aleuticum ‘Japonicum’ ACONITUM arcuatum B&SWJ 774 ADIANTUM aleuticum ‘Miss Sharples’ ACORUS calamus ‘Argenteostriatus’ -

Checklist of Illinois Native Trees
Technical Forestry Bulletin · NRES-102 Checklist of Illinois Native Trees Jay C. Hayek, Extension Forestry Specialist Department of Natural Resources & Environmental Sciences Updated May 2019 This Technical Forestry Bulletin serves as a checklist of Tree species prevalence (Table 2), or commonness, and Illinois native trees, both angiosperms (hardwoods) and gym- county distribution generally follows Iverson et al. (1989) and nosperms (conifers). Nearly every species listed in the fol- Mohlenbrock (2002). Additional sources of data with respect lowing tables† attains tree-sized stature, which is generally to species prevalence and county distribution include Mohlen- defined as having a(i) single stem with a trunk diameter brock and Ladd (1978), INHS (2011), and USDA’s The Plant Da- greater than or equal to 3 inches, measured at 4.5 feet above tabase (2012). ground level, (ii) well-defined crown of foliage, and(iii) total vertical height greater than or equal to 13 feet (Little 1979). Table 2. Species prevalence (Source: Iverson et al. 1989). Based on currently accepted nomenclature and excluding most minor varieties and all nothospecies, or hybrids, there Common — widely distributed with high abundance. are approximately 184± known native trees and tree-sized Occasional — common in localized patches. shrubs found in Illinois (Table 1). Uncommon — localized distribution or sparse. Rare — rarely found and sparse. Nomenclature used throughout this bulletin follows the Integrated Taxonomic Information System —the ITIS data- Basic highlights of this tree checklist include the listing of 29 base utilizes real-time access to the most current and accept- native hawthorns (Crataegus), 21 native oaks (Quercus), 11 ed taxonomy based on scientific consensus. -

Dendrochronology, Fire Regimes
Encyclopedia of Scientific Dating Methods DOI 10.1007/978-94-007-6326-5_76-4 # Springer Science+Business Media Dordrecht 2013 Dendrochronology, Fire Regimes Peter M. Brown* Rocky Mountain Tree-Ring Research, Inc., Fort Collins, CO, USA Definition Use of tree-ring data and methods to reconstruct past fire timing, fire regimes, and fire effects on individuals, communities, and ecosystems. Introduction Fire directly or indirectly affects woody plants in many ways, some of which will leave evidence in age or growth patterns in individual trees or community structure that can be cross-dated using dendrochronological methods. This evidence can be used to reconstruct past fire dates, fire regimes, and fire effects on individuals, communities, and ecosystems (“pyrodendroecology”). Fire regimes are defined as the combination of fire frequency, severity, size, seasonality, and relationships with forcing factors such as climate or changes in human land use. Fire Evidence in Trees and Community Structure A common type of fire evidence is from severe fire that kills all or most trees in an area which opens up space for new trees to establish. Even-aged forest structure is often used as evidence of past lethal fire, also referred to as “stand-replacing” fire. Such evidence provides a minimum date for the fire (i.e., the fire occurred sometime before the innermost dates of the oldest trees), and, by sampling multiple stands in an area, estimates of the fire size can be made based on broader- scale patterns of tree ages. Conversely, multiaged structure is considered to be evidence of less severe fires or other patchy mortality and recruitment events. -

Lauraceae – Laurel Family
LAURACEAE – LAUREL FAMILY Plant: shrubs, trees and some vines Stem: sometimes with aromatic bark Root: Leaves: mostly evergreen, some deciduous; simple with no teeth, often elliptical and somewhat leathery; mostly alternate, less often opposite or whorled; pinnately veined with curved side veins; no stipules; sometimes glandular and aromatic Flowers: perfect but occasionally imperfect (dioecious), regular (actinomorphic); mostly in small clusters along twigs, often yellowish; tepals with 4-6 members in 2 cycles; 3-12-many, but often 9 stamens; ovary superior or inferior,1 carpel, 1 pistil Fruit: berry or drupe, single large seed Other: mostly tropical; locally, sassafras and spicebush common; Dicotyledons Group Genera: 55+ genera; locally Lindera (spice-bush), Persea, Sassafras (sassafras) WARNING – family descriptions are only a layman’s guide and should not be used as definitive LAURACEAE – LAUREL FAMILY [Northern] Spicebush; Lindera benzoin (L.) Blume Sassafras; Sassafras albidum (Nutt.) Nees [Northern] Spicebush USDA Lindera benzoin (L.) Blume Lauraceae (Laurel Family) Oak Openings Metropark, Lucas County, Ohio Notes: shrub; flowers yellow (prior to leaves), in clusters (dioecious); leaves ovate, entire, shiny green above, somewhat whitened below; bark ‘warty’ (pores); winter buds green, stalked, in alternate clusters; twigs hairy or not, often greenish; fruit a red berry or drupe; aromatic; spring [V Max Brown, 2005] Sassafras USDA Sassafras albidum (Nutt.) Nees Lauraceae (Laurel Family) Oak Openings Metropark, Lucas County, Ohio Notes: shrub or tree; flowers small and yellow; leaves alternate, lobed or not (variable), aromatic; bark deeply grooved and ridged; twigs often green (hairy or not – varieties); fruit a blue to purple drupe on red pedicel; spring [V Max Brown, 2005]. -

Cassytha) and Insights Into the Plastid Phylogenomics of Lauraceae
GBE Plastome Evolution in the Sole Hemiparasitic Genus Laurel Dodder (Cassytha) and Insights into the Plastid Phylogenomics of Lauraceae Chung-Shien Wu1,†, Ting-Jen Wang1,†,Chia-WenWu1, Ya-Nan Wang2, and Shu-Miaw Chaw1,* 1Biodiversity Research Center, Academia Sinica, Taipei 11529, Taiwan 2School of Forestry and Resource Conservation, Nation Taiwan University, Taipei 10617, Taiwan *Corresponding author: E-mail: [email protected]. †These authors contributed equally to this work. Accepted: September 6, 2017 Data deposition: This project has been deposited at DDBJ under the accession numbers LC210517, LC212965, LC213014, and LC228240. Abstract To date, little is known about the evolution of plastid genomes (plastomes) in Lauraceae. As one of the top five largest families in tropical forests, the Lauraceae contain many species that are important ecologically and economically. Lauraceous species also provide wonderful materials to study the evolutionary trajectory in response to parasitism because they contain both nonparasitic and parasitic species. This study compared the plastomes of nine Lauraceous species, including the sole hemiparasitic and herbaceous genus Cassytha (laurel dodder; here represented by Cassytha filiformis). We found differential contractions of the canonical inverted repeat (IR), resulting in two IR types present in Lauraceae. These two IR types reinforce Cryptocaryeae and Neocinnamomum— Perseeae–Laureae as two separate clades. Our data reveal several traits unique to Cas. filiformis, including loss of IRs, loss or pseudogenization of 11 ndh and rpl23 genes, richness of repeats, and accelerated rates of nucleotide substitutions in protein- coding genes. Although Cas. filiformis is low in chlorophyll content, our analysis based on dN/dS ratios suggests that both its plastid house-keeping and photosynthetic genes are under strong selective constraints. -

The Occurrence of Red and Yellow Autumn Leaves Explained by Regional Differences in Insolation and Temperature
Review Tansley review The occurrence of red and yellow autumn leaves explained by regional differences in insolation and temperature Authors for correspondence: Susanne S. Renner1 and Constantin M. Zohner2 Susanne S. Renner 1 2 Tel: +49 89 17861 250 Systematic Botany and Mycology, University of Munich (LMU), Menzinger Str. 67, Munich 80638, Germany ; Institute of Email: [email protected] Integrative Biology, Department of Environmental Systems Science, ETH Zurich, Zurich 8092, Switzerland Constantin M. Zohner Email: [email protected] Received: 19 January 2019 Accepted: 24 April 2019 Contents Summary 1464 IV. The adaptive value of colour-changing leaves 1468 I. Introduction 1464 V. Outlook 1469 II. Phylogenetic and geographical occurrence of autumn colour Acknowledgements 1469 change 1465 References 1470 III. Physiological functions of autumnal leaf xanthophylls and anthocyanins 1466 Summary New Phytologist (2019) 224: 1464–1471 Red or yellow autumn leaves have long fascinated biologists, but their geographical doi: 10.1111/nph.15900 concentration in trees in Eastern North America (ENA) has defied evolutionary explanations. In this review, anthocyanins and xanthophylls are discussed in relation to their occurrence in Key words: adaptive explanation, different regions of the Northern Hemisphere, phylogenetic distribution and photoprotective anthocyanins, photo-oxidative damage, function during the breakdown of chlorophylls. Pigments in senescing leaves that intercept regional climates, solar irradiation, incident light and dissipate the absorbed energy extend the time available for nutrient resorption. xanthophylls. Experiments with Arabidopsis have revealed greatest anthocyanin photoprotective function at low temperatures and high light intensities, and high-resolution solar irradiation maps reveal that ENA and Asia receive higher irradiation than does Europe. -

Potential Natural Vegetation and Pre-Anthropic Pollen Records on the Azores
1 Potential natural vegetation and pre-anthropic pollen records on the Azores Islands in a Macaronesian context Valentí Rull1*, Simon E. Connor2,3 & Rui B. Elias4 1Institute of Earth Sciences Jaume Almera (ICTJA-CSIC), 08028 Barcelona, Spain 2School of Geography, University of Melbourne, VIC-3010, Australia 3CIMA-FCT, Universidade do Algarve, Faro 8005-139, Portugal 4Centre for Ecology, Evolution and Environmental Change (CE3C), Universidade dos Açores, Angra do Heroísmo (Açores), Portugal *Corresponding author: Email [email protected] 2 Abstract This paper discusses the concept of potential natural vegetation (PNV) in light of the pollen records available to date for the Macaronesian biogeographical region, with emphasis on the Azores Islands. The classical debate on the convenience or not of the PNV concept has been recently revived in the Canary Islands, where pollen records of pre-anthropic vegetation seemed to strongly disagree with the existing PNV reconstructions. Contrastingly, more recent PNV model outputs from the Azores Islands show outstanding parallelisms with pre-anthropic pollen records, at least in qualitative terms. We suggest the development of more detailed quantitative studies to compare these methodologies as an opportunity for improving the performance of both. PNV modelling may benefit by incorporating empirical data on past vegetation useful for calibration and validation purposes, whereas palynology may improve past reconstructions by minimizing interpretative biases linked to differential pollen production, dispersal and preservation. Keywords: Azores Islands, Canary Islands, Macaronesia, palaeoecology, palynology, potential natural vegetation, pre-anthropic vegetation 3 The idea of potential natural vegetation (PNV) –i.e., the vegetation that would be expected to occur according to the environmental features (notably climate and geology) of a particular area, in the absence of human disturbance or major natural catastrophes- has been the object of intense debate. -

Canary Islands, Spain
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Stapfia Jahr/Year: 2013 Band/Volume: 0099 Autor(en)/Author(s): van den Boom P.P.G. Artikel/Article: Further New or Interesting Lichens and Lichenicolous Fungi of Tenerife (Canary Islands, Spain) 52-60 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.a VAN DEN BOOM • New lichens and lichenicolous fungi from Tenerife STAPFIA 99 (2013): 52–60 Further New or Interesting Lichens and Lichenicolous Fungi of Tenerife (Canary Islands, Spain) P.P.G. VAN DEN BOOM* Abstract: In the presented annotated list, 88 taxa of lichens and lichernicolous fungi are additional records for the island Tenerife, of which 19 are new to the Canary Islands: Abrothallus aff. secedens, Anisomeridium robusta, Arthonia elegans, Buellia abstracta, B. fusca, Caloplaca phlogina, Endococcus pseudocarpus, Lecania brunonis, Lecanographa lyncea, Lecanora persimilis, L. subsaligna, Physcia atrostriata, P. sore- diosa, Plectocarpon nashii, Porina hoehneliana, Protopannaria pezizoides, Sarcopyrenia bacillosa, Scoli- ciosporum gallurae and Toninia talparum. Furthermore Bacidina pseudoisidiata and Micarea canariensis are newly described. Zusammenfassung: Eine annotierte Liste von 88 Flechten und lichenikolen Pilzen wird präsentiert mit wei- teren Funden für die Insel Teneriffa, von denen 19 neu für die Kanarischen Inseln sind: Abrothallus secedens, Anisomeridium robusta, Arthonia elegans, Buellia abstracta, B. fusca, Caloplaca phlogina, Endococcus pseudocarpus, Lecania brunonis, Lecanographa lyncea, Lecanora persimilis, L. subsaligna, Physcia atros- triata, P. sorediosa, Plectocarpon nashii, Porina hoehneliana, Protopannaria pezizoides, Sarcopyrenia bacil- losa, Scoliciosporum gallurae und Toninia talparum. Weiters werden Bacidina pseudoisidiata und Micarea canariensis neu beschrieben. Key words: diversity in lichens and lichenicolous fungi, new species, new records, ecology, Macaronesia. -

An Experimental Evaluation of Fire History Reconstruction Using Dendrochronology in White Oak (Quercus Alba)
1 An experimental evaluation of fire history reconstruction using dendrochronology in white oak (Quercus alba) Ryan W. McEwan, Todd F. Hutchinson, Robert D. Ford, and Brian C. McCarthy Abstract: Dendrochronological analysis of fire scars on tree cross sections has been critically important for understanding historical fire regimes and has influenced forest management practices. Despite its value as a tool for understanding histor- ical ecosystems, tree-ring-based fire history reconstruction has rarely been experimentally evaluated. To examine the effi- cacy of dendrochronological analysis for detecting fire occurrence in oak forests, we analyzed tree cross sections from sites in which prescribed fires had been recently conducted. The first fire in each treatment unit created a scar in at least one sample, but the overall percentage of samples containing scars in fire years was low (12%). We found that scars were cre- ated by 10 of the 15 prescribed fires, and the five undetected fires all occurred in sites where fire had occurred the previous year. Notably, several samples contained scars from known fire-free periods. In summary, our data suggest that tree-ring analysis is a generally effective tool for reconstructing historical fire regimes, although the following points of uncertainty were highlighted: (i) consecutive annual burns may not create fire scars and (ii) wounds that are morphologically indistin- guishable from fire scars may originate from nonfire sources. R6um6 : L'analyse dendrochronologique des cicatrices de feu sur des sections radiales de tronc d'arbre a it6 d'une im- portance cruciale pour comprendre les regimes des feux pass& et a influenck les pratiques d'amtnagement forestier. -

Towards an Updated Checklist of the Libyan Flora
Towards an updated checklist of the Libyan flora Article Published Version Creative Commons: Attribution 3.0 (CC-BY) Open access Gawhari, A. M. H., Jury, S. L. and Culham, A. (2018) Towards an updated checklist of the Libyan flora. Phytotaxa, 338 (1). pp. 1-16. ISSN 1179-3155 doi: https://doi.org/10.11646/phytotaxa.338.1.1 Available at http://centaur.reading.ac.uk/76559/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . Published version at: http://dx.doi.org/10.11646/phytotaxa.338.1.1 Identification Number/DOI: https://doi.org/10.11646/phytotaxa.338.1.1 <https://doi.org/10.11646/phytotaxa.338.1.1> Publisher: Magnolia Press All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online Phytotaxa 338 (1): 001–016 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2018 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.338.1.1 Towards an updated checklist of the Libyan flora AHMED M. H. GAWHARI1, 2, STEPHEN L. JURY 2 & ALASTAIR CULHAM 2 1 Botany Department, Cyrenaica Herbarium, Faculty of Sciences, University of Benghazi, Benghazi, Libya E-mail: [email protected] 2 University of Reading Herbarium, The Harborne Building, School of Biological Sciences, University of Reading, Whiteknights, Read- ing, RG6 6AS, U.K. -
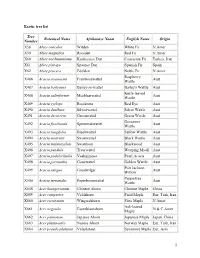
1 Exotic Tree List Tree Number Botanical Name Afrikaanse Naam
Exotic tree list Tree Botanical Name Afrikaanse Naam English Name Origin Number X58 Abies concolor Witden White Fir N.Amer X59 Abies magnifica Rooiden Red Fir N.Amer X60 Abies nordmanniana Kaukasiese Den Caucasian Fir Turkey, Iran X61 Abies pinsapo Spaanse Den Spanish Fir Spain X62 Abies procera Edelden Noble Fir N.Amer Raspberry X486 Acacia acuminata Frambosewattel Aust Wattle X487 Acacia baileyana Bailey-se-wattel Bailey's Wattle Aust Knife-leaved X488 Acacia cultriformis Mesblaarwattel Aust Wattle X489 Acacia cyclops Rooikrans Red Eye Aust X490 Acacia dealbata Silwerwattel Silver Wattle Aust X491 Acacia decurrens Groenwattel Green Wattle Aust Gossamer X492 Acacia floribunda Spinnerakwattel Aust Wattle X493 Acacia longifolia Bleekwattel Sallow Wattle Aust X494 Acacia mearnsii Swartwattel Black Wattle Aust X495 Acacia melanoxylon Swarthout Blackwood Aust X496 Acacia pendula Treurwattel Weeping Myall Aust X497 Acacia podalyriifolia Vaalmimosa Pearl Acacia Aust X498 Acacia pycnantha Gouewattel Golden Wattle Aust Port Jackson X499 Acacia saligna Goudwilger Aust Willow Peppertree X500 Acacia terminalis Peperboomwattel Aust Wattle X658 Acer buergerianum Chinese Ahorn Chinese Maple China X659 Acer campestre Veldahorn Field Maple Eur, Turk, Iran X660 Acer circinatum Wingerdahorn Vine Maple N Amer Ash-leaved X661 Acer negundo Essenblaarahorn N & C Amer Maple X662 Acer palmatum Japanse Ahorn Japanese Maple Japan, China X663 Acer platanoides Noorse Ahorn Norway Maple Eur, Turk, Iran X664 Acer pseudo-platanus Valsplataan Sycamore Maple Eur, Asia