Manufacturer Patient Assistance Programs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigator Initiated Study IRB-29839 an Open-Label Pilot Study To
Investigator Initiated Study IRB-29839 An open-label pilot study to evaluate the efficacy and safety of a combination treatment of Sonidegib and BKM120 for the treatment of advanced basal cell carcinomas Version 05 September 2016 NCT02303041 DATE: 12Dec2018 1 Coordinating Center Stanford Cancer Center 875 Blake Wilbur Drive Stanford, CA 94305 And 450 Broadway, MC 5334 Redwood City, CA 94603 Protocol Director and Principal Investigator Anne Lynn S Chang, MD, Director of Dermatological Clinical Trials 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Co-Investigator Anthony Oro, MD PhD 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Biostatistician Shufeng Li, MS 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Study Coordinator Ann Moffat 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] 2 Table of Contents 1 Background ................................................................. 7 1.1 Disease Background ..................................................... 7 1.2 Hedgehog Pathway and mechanism of action ............................... 7 1.3 PI3K Pathway and mechanism of action ................................... 9 1.4 Sonidegib Compound Information ............ Error! Bookmark not defined. 1.4.1 Preclinical Studies for Sonidegib ....................................................................11 1.4.2 Muscular system...............................................................................................13 1.4.3 Skeletal system ................................................................................................13 -

Tumour-Agnostic Therapy for Pancreatic Cancer and Biliary Tract Cancer
diagnostics Review Tumour-Agnostic Therapy for Pancreatic Cancer and Biliary Tract Cancer Shunsuke Kato Department of Clinical Oncology, Juntendo University Graduate School of Medicine, 2-1-1, Hongo, Bunkyo-ku, Tokyo 113-8421, Japan; [email protected]; Tel.: +81-3-5802-1543 Abstract: The prognosis of patients with solid tumours has remarkably improved with the develop- ment of molecular-targeted drugs and immune checkpoint inhibitors. However, the improvements in the prognosis of pancreatic cancer and biliary tract cancer is delayed compared to other carcinomas, and the 5-year survival rates of distal-stage disease are approximately 10 and 20%, respectively. How- ever, a comprehensive analysis of tumour cells using The Cancer Genome Atlas (TCGA) project has led to the identification of various driver mutations. Evidently, few mutations exist across organs, and basket trials targeting driver mutations regardless of the primary organ are being actively conducted. Such basket trials not only focus on the gate keeper-type oncogene mutations, such as HER2 and BRAF, but also focus on the caretaker-type tumour suppressor genes, such as BRCA1/2, mismatch repair-related genes, which cause hereditary cancer syndrome. As oncogene panel testing is a vital approach in routine practice, clinicians should devise a strategy for improved understanding of the cancer genome. Here, the gene mutation profiles of pancreatic cancer and biliary tract cancer have been outlined and the current status of tumour-agnostic therapy in these cancers has been reported. Keywords: pancreatic cancer; biliary tract cancer; targeted therapy; solid tumours; driver mutations; agonist therapy Citation: Kato, S. Tumour-Agnostic Therapy for Pancreatic Cancer and 1. -

PRIOR AUTHORIZATION CRITERIA for APPROVAL Initial Evaluation Target Agent(S) Will Be Approved When ONE of the Following Is Met: 1
Self-Administered Oncology Agents Through Preferred Prior Authorization Program Summary FDA APPROVED INDICATIONS3-104 Please reference individual agent product labeling. CLINICAL RATIONALE For the purposes of the Self -Administered Oncology Agents criteria, indications deemed appropriate are those approved in FDA labeling and/or supported by NCCN Drugs & Biologics compendia with a category 1 or 2A recommendation, AHFS, or DrugDex with level of evidence of 1 or 2A. SAFETY3-104 Agent(s) Contraindication(s) Afinitor/Afinitor Disperz Hypersensitivity to everolimus, to other rapamycin (everolimus) derivatives None Alecensa (alectinib) Alunbrig (brigatinib) None Ayvakit (avapritinib) None Balversa (erdafitinib) None Hypersensitivity to bosutinib Bosulif (bosutinib) Braftovi (encorafenib) None Brukinsa (zanubrutinib) None Cabometyx None (cabozantinib) Calquence None (acalabrutinib) Caprelsa Congenital long QT syndrome (vandetanib) Cometriq None (cabozantinib) Copiktra (duvelisib) None Cotellic (cobimetinib) None Daurismo (glasdegib) None None Erivedge (vismodegib) Erleada (apalutamide) Pregnancy None Farydak (panobinostat) Fotivda (tivozanib) None Gavreto (pralsetinib) None None Gilotrif (afatinib) Gleevec None (imatinib) Hycamtin Severe hypersensitivity to topotecan (topotecan) None Ibrance (palbociclib) KS_PS_SA_Oncology_PA_ProgSum_AR1020_r0821v2 Page 1 of 19 © Copyright Prime Therapeutics LLC. 08/2021 All Rights Reserved Effective: 10/01/2021 Agent(s) Contraindication(s) None Iclusig (ponatinib) Idhifa (enasidenib) None Imbruvica (ibrutinib) -

Pharmacy Pre-Authorization Criteria
PHARMACY PRE-AUTHORIZATION CRITERIA DRUG (S) Oncology Medications: Sprycel (dasatinib) Afinitor (everolimus) Stivarga (regorafenib) Caprelsa (vandetanib) Sutent (sunitinib) Gilotrif (afatinib dimaleate) Tarceva (erlotinob) Gleevec (imatinib mesylate) Temodar (temozolomide) Ibrance (palbociclib) Tepadina (thiotepa) Idhifa (enasidenib) Thalomid (thalidomide) Iressa (gefitinib) Vantas (histrelin acetate) Kisqali (ribociclib) Venclexta (venetoclax) Lenvima (lenvatinib) Verzenio (abemaciclib) Nerlynx (neratinib) Vidaza (azacitidine) Nexavar (sorafenib) Votrient (pazopanib) Ninlaro (ixazomib) Xalkori (crizotinib) Odomzo (sonidegib) Xeloda (capecitabine) Zydelig (idelalisib) Zykadia (certinib) Zytiga (abiraterone acetate) PHARMACY PRE-AUTHORIZATION CRITERIA POLICY # 21107 CRITERIA The above medications are covered when the following criteria are met: 1. Ordered by an oncologist or hematologist AND 2. All FDA Approved Indications OR 3. Chemo agent is listed in an accepted Compendia for treatment of cancer type, OR - National Comprehensive Cancer Network (NCCN) Drugs and Biologics Compendium, level of evidence 1, 2A, or 2B - Thomson Micromedex DrugDex - Chemo agent is recommended for cancer type in formal clinical studies published in at least 2 peer reviewed professional medical journals, published in the United States or Great Britain Coverage Duration: Initial: 3 months Continuation: 6 months. Afinitor, Gilotrif, Gleevec, Ibrance, Idhifa, Iressa, Kisqali, Lenvima, Nexavar, Ninlaro, Odomzo, Sprycel, Stivarga, Sutent, Tarceva, Temodar, Thalomid, -

211192Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 211192Orig1s000 RISK ASSESSMENT and RISK MITIGATION REVIEW(S) Division of Risk Management (DRISK) Office of Medication Error Prevention and Risk Management (OMEPRM) Office of Surveillance and Epidemiology (OSE) Center for Drug Evaluation and Research (CDER) Application Type NDA Application Number 211192 PDUFA Goal Date August 21, 2018 OSE RCM # 2017-2612; 2017-2614 Reviewer Name(s) Till Olickal, Ph.D., Pharm.D. Team Leader Elizabeth Everhart, MSN, RN, ACNP Division Director Cynthia LaCivita, Pharm.D. Review Completion Date June 6, 2018 Subject Review to determine if a REMS is necessary Established Name Ivosidenib Trade Name Tibsovo Name of Applicant Agios Pharmaceuticals, Inc. Therapeutic Class Isocitrate dehydrogenase-1 inhibitor Formulation(s) 250 mg tablet Dosing Regimen 500 mg orally once daily until disease progression or unacceptable toxicity. 1 Reference ID: 4273770 Table of Contents EXECUTIVE SUMMARY ......................................................................................................................................................... 3 1 Introduction ..................................................................................................................................................................... 3 2 Background ...................................................................................................................................................................... 3 2.1 Product Information .......................................................................................................................................... -
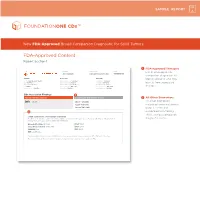
FDA-Approved Content Report Section 1
SAMPLE REPORT New FDA-Approved Broad Companion Diagnostic for Solid Tumors FDA-Approved Content Report Section 1 1 FDA-Approved Therapies PATIENT TUMOR TYPE TRF# List of FDA-approved Jane Sample Lung adenocarcinoma TRFXXXXXX companion diagnostics to PATIENT PHYSICIAN SPECIMEN identify patients who may DISEASE Lung adenocarcinoma ORDERING PHYSICIAN Not Given SPECIMEN SITE Not Given NAME Not Given MEDICAL FACILITY Not Given SPECIMEN ID Not Given benefi t from associated DATE OF BIRTH Not Given ADDITIONAL RECIPIENT Not Given SPECIMEN TYPE Not Given SEX Female MEDICAL FACILITY ID Not Given DATE OF COLLECTION Not Given therapies MEDICAL RECORD # Not Given PATHOLOGIST Not Given SPECIMEN RECEIVED Not Given CDx Associated Findings 1 GENOMIC FINDINGS DETECTED FDA-APPROVED THERAPEUTIC OPTIONS 2 All Other Biomarkers EGFR L858R Gilotrif® (Afatinib) All other biomarkers, Iressa® (Gefitinib) including tumor mutational Tarceva® (Erlotinib) burden (TMB) and 2 microsatellite instability (MSI), without companion OTHER ALTERATIONS & BIOMARKERS IDENTIFIED Results reported in this section are not prescriptive or conclusive for labeled use of any specific therapeutic product. See diagnostic claims professional services section for additional information. Microsatellite Status MS-Stable PTCH1 T416S Tumor Mutation Burden 11 Muts/Mb RBM10 Q494* CDKN2A/B loss TP53 R267P EGFR amplification § Refer to appendix for limitation statements related to detection of any copy number alterations, gene rearrangements, MSI or TMB result in this section. Please refer to appendix -

Horizon Scanning Status Report June 2019
Statement of Funding and Purpose This report incorporates data collected during implementation of the Patient-Centered Outcomes Research Institute (PCORI) Health Care Horizon Scanning System, operated by ECRI Institute under contract to PCORI, Washington, DC (Contract No. MSA-HORIZSCAN-ECRI-ENG- 2018.7.12). The findings and conclusions in this document are those of the authors, who are responsible for its content. No statement in this report should be construed as an official position of PCORI. An intervention that potentially meets inclusion criteria might not appear in this report simply because the horizon scanning system has not yet detected it or it does not yet meet inclusion criteria outlined in the PCORI Health Care Horizon Scanning System: Horizon Scanning Protocol and Operations Manual. Inclusion or absence of interventions in the horizon scanning reports will change over time as new information is collected; therefore, inclusion or absence should not be construed as either an endorsement or rejection of specific interventions. A representative from PCORI served as a contracting officer’s technical representative and provided input during the implementation of the horizon scanning system. PCORI does not directly participate in horizon scanning or assessing leads or topics and did not provide opinions regarding potential impact of interventions. Financial Disclosure Statement None of the individuals compiling this information have any affiliations or financial involvement that conflicts with the material presented in this report. Public Domain Notice This document is in the public domain and may be used and reprinted without special permission. Citation of the source is appreciated. All statements, findings, and conclusions in this publication are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI) or its Board of Governors. -

209606Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 209606Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review NDA Multidisciplinary Review and Evaluation Application Number(s) NDA 209606 Application Type Original 505(b)(1) Priority or Standard Priority Submit Date(s) December 30, 2016 Received Date(s) December 30, 2016 PDUFA Goal Date August 30, 2017 Division/Office DHP/OHOP Review Completion Date July 28, 2017 Applicant Celgene Corporation Established Name Enasidenib (Proposed) Trade Name IDHIFA® Pharmacologic Class Isocitrate dehydrogenase 2 inhibitor Formulation(s) Tablets, 50mg and 100mg Dosing Regimen 100 mg once daily Applicant Proposed IDHIFA is indicated for the treatment of patients with relapsed or Indication(s)/Population(s) refractory acute myeloid leukemia with an IDH2 mutation Recommendation on Regular approval Regulatory Action Recommended IDHIFA is an isocitrate dehydrogenase-2 inhibitor indicated for the Indication(s)/Population(s) treatment of adult patients with relapsed or refractory acute myeloid leukemia with an isocitrate dehydrogenase-2 mutation as detected by an FDA-approved test. Reference ID: 4131433 Multidisciplinary Review and Evaluation NDA 209606 IDHIFA® (enasidenib) Table of Contents Table of Contents ........................................................................................................................... 2 Table of Tables .............................................................................................................................. -

Enasidenib Drives Human Erythroid Differentiation Independently of Isocitrate Dehydrogenase 2
The Journal of Clinical Investigation CONCISE COMMUNICATION Enasidenib drives human erythroid differentiation independently of isocitrate dehydrogenase 2 Ritika Dutta,1,2 Tian Yi Zhang,1,2 Thomas Köhnke,1 Daniel Thomas,1 Miles Linde,1 Eric Gars,1 Melissa Stafford,1 Satinder Kaur,1 Yusuke Nakauchi,1 Raymond Yin,1 Armon Azizi,1 Anupama Narla,3 and Ravindra Majeti1,2 1Department of Medicine, Division of Hematology, Cancer Institute, and Institute for Stem Cell Biology and Regenerative Medicine, Stanford University, Stanford, California, USA. 2Stanford School of Medicine, Stanford, California, USA. 3Department of Pediatrics, Division of Hematology/Oncology, Stanford University, Stanford, California, USA. Cancer-related anemia is present in more than 60% of newly diagnosed cancer patients and is associated with substantial morbidity and high medical costs. Drugs that enhance erythropoiesis are urgently required to decrease transfusion rates and improve quality of life. Clinical studies have observed an unexpected improvement in hemoglobin and RBC transfusion- independence in patients with acute myeloid leukemia (AML) treated with the isocitrate dehydrogenase 2 (IDH2) mutant- specific inhibitor enasidenib, leading to improved quality of life without a reduction in AML disease burden. Here, we demonstrate that enasidenib enhanced human erythroid differentiation of hematopoietic progenitors. The phenomenon was not observed with other IDH1/2 inhibitors and occurred in IDH2-deficient CRISPR-engineered progenitors independently of D-2-hydroxyglutarate. The effect of enasidenib on hematopoietic progenitors was mediated by protoporphyrin accumulation, driving heme production and erythroid differentiation in committed CD71+ progenitors rather than hematopoietic stem cells. Our results position enasidenib as a promising therapeutic agent for improvement of anemia and provide the basis for a clinical trial using enasidenib to decrease transfusion dependence in a wide array of clinical contexts. -

An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib Christina Danial, Kavita Y
Published OnlineFirst November 6, 2015; DOI: 10.1158/1078-0432.CCR-15-1588 Clinical Trial Brief Report Clinical Cancer Research An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib Christina Danial, Kavita Y. Sarin, Anthony E. Oro, and Anne Lynn S. Chang Abstract Purpose: To assess the tumor response to the smoothened sive disease with sonidegib. Three patients experienced stable (SMO) inhibitor, sonidegib (LDE225), in patients with an disease and discontinued sonidegib either due to adverse events advanced basal cell carcinoma (BCC) resistant to treatment with (n ¼ 1) or due to election for surgery (n ¼ 2). The response of one vismodegib (GDC0449). patient was not evaluable. SMO mutations with in vitro data Experimental Design: Nine patients with an advanced suggesting resistance to Hh pathway inhibition were identified BCC that was previously resistant to treatment with vismode- in 5 patients, and none of these patients experienced responses gib were given sonidegib in this investigational, open- while on sonidegib. label study. Tumor response was determined using the Conclusion: Patients with advanced BCCs that were response evaluation criteria in solid tumors. SMO mutations previously resistant to treatment with vismodegib similarly were identified using biopsy samples from the target BCC demonstrated treatment resistance with sonidegib. Patients location. who have developed treatment resistance to an SMO inhibitor Results: The median duration of treatment with sonidegib was may continue to experience tumor progression in response to 6 weeks (range, 3–58 weeks). Five patients experienced progres- other SMO inhibitors. Clin Cancer Res; 1–5. Ó2015 AACR. Introduction Sonidegib (LDE225) is a new SMO inhibitor approved in 2015 by the FDA for locally advanced BCCs. -

Imatinib (Gleevec™)
Biologicals What Are They? When Did All of this Happen? There are Clear Benefits. Are there also Risks? Brian J Ward Research Institute of the McGill University Health Centre Global Health, Immunity & Infectious Diseases Grand Rounds – March 2016 Biologicals Biological therapy involves the use of living organisms, substances derived from living organisms, or laboratory-produced versions of such substances to treat disease. National Cancer Institute (USA) What Effects Do Steroids Have on Immune Responses? This is your immune system on high dose steroids projects.accessatlanta.com • Suppress innate and adaptive responses • Shut down inflammatory responses in progress • Effects on neutrophils, macrophages & lymphocytes • Few problems because use typically short-term Virtually Every Cell and Pathway in Immune System ‘Target-able’ (Influenza Vaccination) Reed SG et al. Nature Medicine 2013 Nakaya HI et al. Nature Immunology 2011 Landscape - 2013 Antisense (30) Cell therapy (69) Gene Therapy (46) Monoclonal Antibodies (308) Recombinant Proteins (93) Vaccines (250) Other (81) http://www.phrma.org/sites/default/files/pdf/biologicsoverview2013.pdf Therapeutic Category Drugs versus Biologics Patented Ibuprofen (Advil™) Generic Ibuprofen BioSimilars/BioSuperiors ? www.drugbank.ca Patented Etanercept (Enbrel™) BioSimilar Etanercept Etacept™ (India) Biologics in Cancer Therapy Therapeutic Categories • Hormonal Therapy • Monoclonal antibodies • Cytokine therapy • Classical vaccine strategies • Adoptive T-cell or dendritic cells transfer • Oncolytic -

Revised 11/25/2019 GEORGIA MEDICAID FEE-FOR-SERVICE
GEORGIA MEDICAID FEE-FOR-SERVICE ONCOLOGY, ORAL - HEMATOLOGIC PA SUMMARY Preferred Non-Preferred Bosulif (bosutinib) Purixan (mercaptopurine suspension) Calquence (acalabrutinib) Farydak (panobinostat) Iclusig (ponatinib) Idhifa (enasidenib) Imbruvica (ibrutinib) Mercaptopurine tablets generic* Ninlaro (ixazomib) Pomalyst (pomalidomide) Revlimid (lenalidomide)* Rydapt (midostaurin) Sprycel (dasatinib) Tasigna (nilotinib) Thalomid (thalidomide)* Venclexta (venetoclax) Xpovio (selinexor) Zolinza (vorinosta) Zydelig (idelalisib) *PA not required LENGTH OF AUTHORIZATION: 1 Year NOTES: ▪ Mercaptopurine generic, Revlimid and Thalomid do not require prior authorization. ▪ Special consideration taken for members with stage IV advanced metastatic cancer. PA CRITERIA: Bosulif ❖ Approvable for members with a diagnosis of chronic-phase Philadelphia chromosome- positive (Ph+) chronic myelogenous leukemia (CML). ❖ Approvable for members with a diagnosis of accelerated- or blast-phase Ph+ CML who are resistant or intolerant to imatinib (Gleevec), dasatinib (Sprycel) or nilotinib (Tasigna). ❖ Approvable for members with a diagnosis of Ph+ acute lymphoblastic leukemia (ALL) who are resistant or intolerant to imatinib (Gleevec), dasatinib (Sprycel) or nilotinib (Tasigna). Calquence ❖ Approvable for members with a diagnosis of mantle cell lymphoma (MCL) who have received at least one prior therapy. Farydak ❖ Approvable for members with a diagnosis of multiple myeloma who have been previously treated with at least 2 prior therapies, including bortezomib (Velcade) and an Revised 11/25/2019 immunomodulatory agent (lenalidomide [Revlimid], thalidomide [Thalomid], pomalidomide [Pomalyst]) ❖ Farydak must be given in combination with bortezomib (Velcade) and dexamethasone, with lenalidomide (Revlimid) and dexamethasone or with carfilzomib [Kyprolis]. Iclusig ❖ Approvable for members with a diagnosis of chronic (not newly diagnosed), accelerated or blast-phase CML who are resistant or intolerant to imatinib (Gleevec), bosutinib (Bosulif), dasatinib (Sprycel) and nilotinib (Tasigna).