Imatinib (Gleevec™)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potential High-Impact Interventions Report Priority Area 02: Cancer
AHRQ Healthcare Horizon Scanning System – Potential High-Impact Interventions Report Priority Area 02: Cancer Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. HHSA290201000006C Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 December 2012 Statement of Funding and Purpose This report incorporates data collected during implementation of the Agency for Healthcare Research and Quality (AHRQ) Healthcare Horizon Scanning System by ECRI Institute under contract to AHRQ, Rockville, MD (Contract No. HHSA290201000006C). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. This report’s content should not be construed as either endorsements or rejections of specific interventions. As topics are entered into the System, individual topic profiles are developed for technologies and programs that appear to be close to diffusion into practice in the United States. Those reports are sent to various experts with clinical, health systems, health administration, and/or research backgrounds for comment and opinions about potential for impact. The comments and opinions received are then considered and synthesized by ECRI Institute to identify interventions that experts deemed, through the comment process, to have potential for high impact. Please see the methods section for more details about this process. This report is produced twice annually and topics included may change depending on expert comments received on interventions issued for comment during the preceding 6 months. -

Investigator Initiated Study IRB-29839 an Open-Label Pilot Study To
Investigator Initiated Study IRB-29839 An open-label pilot study to evaluate the efficacy and safety of a combination treatment of Sonidegib and BKM120 for the treatment of advanced basal cell carcinomas Version 05 September 2016 NCT02303041 DATE: 12Dec2018 1 Coordinating Center Stanford Cancer Center 875 Blake Wilbur Drive Stanford, CA 94305 And 450 Broadway, MC 5334 Redwood City, CA 94603 Protocol Director and Principal Investigator Anne Lynn S Chang, MD, Director of Dermatological Clinical Trials 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Co-Investigator Anthony Oro, MD PhD 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Biostatistician Shufeng Li, MS 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Study Coordinator Ann Moffat 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] 2 Table of Contents 1 Background ................................................................. 7 1.1 Disease Background ..................................................... 7 1.2 Hedgehog Pathway and mechanism of action ............................... 7 1.3 PI3K Pathway and mechanism of action ................................... 9 1.4 Sonidegib Compound Information ............ Error! Bookmark not defined. 1.4.1 Preclinical Studies for Sonidegib ....................................................................11 1.4.2 Muscular system...............................................................................................13 1.4.3 Skeletal system ................................................................................................13 -

WO 2018/223101 Al 06 December 2018 (06.12.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/223101 Al 06 December 2018 (06.12.2018) W !P O PCT (51) International Patent Classification: (71) Applicant: JUNO THERAPEUTICS, INC. [US/US]; 400 A 61K 35/1 7 (20 15.0 1) A 61P 35/00 (2006 .0 1) Dexter Ave. N., Suite 1200, Seattle, WA 98109 (US). (21) International Application Number: (72) Inventor: ALBERTSON, Tina; 400 Dexter Ave. N., Suite PCT/US2018/035755 1200, Seattle, WA 98109 (US). (22) International Filing Date: (74) Agent: AHN, Sejin et al; Morrison & Foerster LLP, 1253 1 0 1 June 2018 (01 .06.2018) High Bluff Drive, Suite 100, San Diego, CA 92130-2040 (US). (25) Filing Language: English (81) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of national protection available): AE, AG, AL, AM, (30) Priority Data: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, 62/5 14,774 02 June 2017 (02.06.2017) US CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, 62/5 15,530 05 June 2017 (05.06.2017) US DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, 62/521,366 16 June 2017 (16.06.2017) u s HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, 62/527,000 29 June 2017 (29.06.2017) u s KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, 62/549,938 24 August 2017 (24.08.2017) u s MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 62/580,425 0 1 November 2017 (01 .11.2017) u s OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 62/593,871 0 1 December 2017 (01 .12.2017) u s SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 62/596,764 08 December 2017 (08.12.2017) u s TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Pan-Canadian Pricing Alliance: Completed Negotiations
Pan-Canadian Pricing Alliance: Completed Negotiations As of August 31, 2014 46 joint negotiations have been completed** for the following drugs and indications: Drug Product Indication/Use Brand Name (Generic Name) Please refer to individual jurisdictions for specific reimbursement criteria Adcetris (brentuximab) Used to treat lymphoma Afinitor (everolimus) Used to treat pancreatic neuroendocrine tumours, and to treat breast cancer Alimta (pemetrexed) Used to treat lung cancer (new indication) Used with ASA to prevent atherothrombotic events in patients with acute Brilinta (ticagrelor)† coronary syndrome Dificid Used to treat Clostridium difficile Used with ASA to prevent atherothrombotic events in patients with acute Effient (prasugrel) coronary syndrome Used to prevent strokes in patients with atrial fibrillation, and to prevent deep Eliquis (apixaban) vein thrombosis Erivedge (vismodegib) Used to treat metastatic basal cell carcinoma Esbriet (pirfenidone)* Used to treat idiopathic pulmonary fibrosis Galexos (simeprevir)* Used to treat chronic hepatitis C Genotype 1 infection Gilenya (fingolimod)† Used to treat relapsing-remitting multiple sclerosis Giotrif (afatinib) Used to treat EGFR mutation positive, advanced non-small cell lung cancer Halaven (eribulin) Used to treat breast cancer Inlyta Used to treat metastatic renal cell carcinoma Jakavi (ruxolitinib) Used to treat intermediate to high risk myelofibrosis Kadcyla (trastuzumab emtansine) Used to treat HER-2 positive metastatic breast cancer Kalydeco (ivacaftor) Used to treat cystic -

ADC Therapeutics SA (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 For the month of July, 2020. Commission File Number: 001-39071 ADC Therapeutics SA (Exact name of registrant as specified in its charter) Biopôle Route de la Corniche 3B 1066 Epalinges Switzerland (Address of principal executive office) Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F: Form 20-F ☒ Form 40-F Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐ Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐ SIGNATURE Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized. ADC Therapeutics SA Date: July 6, 2020 By: /s/ Dominique Graz Name: Dominique Graz Title: General Counsel EXHIBIT INDEX Exhibit No. Description 99.1 Press release dated July 6, 2020 Exhibit 99.1 ADC Therapeutics Announces U.S. Food and Drug Administration Has Lifted Partial Clinical Hold on Pivotal Phase 2 Clinical Trial of Camidanlumab Tesirine Lausanne, Switzerland — July 6, 2020 — ADC Therapeutics SA (NYSE:ADCT), a clinical-stage oncology-focused biotechnology company leading the development and commercialization of next-generation antibody drug conjugates (ADCs) with highly potent and targeted pyrrolobenzodiazepine (PBD) dimer technology, today announced that the U.S. -

Comparative Effectiveness of Pembrolizumab Vs. Nivolumab In
www.nature.com/scientificreports OPEN Comparative efectiveness of pembrolizumab vs. nivolumab in patients with recurrent or advanced NSCLC Pengfei Cui1,2,4, Ruixin Li2,4, Ziwei Huang3,2,4, Zhaozhen Wu3,2, Haitao Tao2, Sujie Zhang2 & Yi Hu1,2,3* The efcacies of pembrolizumab and nivolumab have never been directly compared in a real-world study. Therefore, we sought to retrospectively evaluate the objective response rate (ORR) and the progression-free survival (PFS) of patients with recurrent or advanced non-small cell lung cancer (NSCLC) in a real-world setting. This study included patients with recurrent or advanced NSCLC diagnosed between September 1, 2015 and August 31, 2019, who were treated with programmed cell death 1 (PD-1) inhibitors at the Cancer Center of the Chinese People’s Liberation Army. PFS was estimated for each treatment group using Kaplan–Meier curves and log-rank tests. The multivariate analysis of PFS was performed with Cox proportional hazards regression models. A total of 255 patients with advanced or recurrent NSCLC treated with PD-1 inhibitors were identifed. The ORR was signifcantly higher in the pembrolizumab group than in the nivolumab group, while PFS was not signifcantly diferent between the two groups. Subgroup analysis showed that the ORR was signifcantly higher for pembrolizumab than for nivolumab in patients in the frst-line therapy subgroup and in those in the combination therapy as frst-line therapy subgroup. Survival analysis of patients receiving combination therapy as second- or further-line therapy showed that nivolumab had better efcacy than pembrolizumab. However, the multivariate analysis revealed no signifcant diference in PFS between patients treated with pembrolizumab and those treated with nivolumab regardless of the subgroup. -

Phase I-II Study of Ruxolitinib (INCB18424) for Patients With
Protocol Page Phase I-II Study of Ruxolitinib (INCB18424) for Patients with Chronic Myeloid Leukemia (CML) with Minimal Residual Disease While on Therapy with Tyrosine Kinase Inhibitors 2012-0697 Core Protocol Information Short Title Ruxolitinib for CML with MRD Study Chair: Jorge Cortes Additional Contact: Allison Pike Rachel R. Abramowicz Leukemia Protocol Review Group Additional Memo Recipients: Recipients List OPR Recipients (for OPR use only) None Study Staff Recipients None Department: Leukemia Phone: 713-794-5783 Unit: 428 Study Manager: Allison Pike Full Title: Phase I-II Study of Ruxolitinib (INCB18424) for Patients with Chronic Myeloid Leukemia (CML) with Minimal Residual Disease While on Therapy with Tyrosine Kinase Inhibitors Public Description: Protocol Type: Standard Protocol Protocol Phase: Phase I/Phase II Version Status: Terminated 10/03/2019 Version: 24 Document Status: Saved as "Final" Submitted by: Rachel R. Abramowicz--5/7/2019 9:28:03 AM OPR Action: Accepted by: Amber M. Cumpian -- 5/8/2019 5:48:18 PM Which Committee will review this protocol? The Clinical Research Committee - (CRC) Protocol Body 2012-0697 April 6, 2015 Page 1 of 41 Phase I-II Study of Ruxolitinib (INCB18424) for Patients with Chronic Myeloid Leukemia (CML) with Minimal Residual Disease While on Therapy with Tyrosine Kinase Inhibitors Principal Investigator Jorge Cortes, MD Department of Leukemia MD Anderson Cancer Center 1515 Holcombe Blvd., Unit 428 Houston, TX 77030 (713)792-7305 Study Product: Ruxolitinib Protocol Number: 2012-0697 Coordinating Center: MD Anderson Cancer Center 1515 Holcombe Blvd., Unit 428 Houston, TX 77030 2012-0697 April 6, 2015 Page 2 of 41 1. -

Cutaneous Adverse Effects of Biologic Medications
REVIEW CME MOC Selena R. Pasadyn, BA Daniel Knabel, MD Anthony P. Fernandez, MD, PhD Christine B. Warren, MD, MS Cleveland Clinic Lerner College Department of Pathology Co-Medical Director of Continuing Medical Education; Department of Dermatology, Cleveland Clinic; of Medicine of Case Western and Department of Dermatology, W.D. Steck Chair of Clinical Dermatology; Director of Clinical Assistant Professor, Cleveland Clinic Reserve University, Cleveland, OH Cleveland Clinic Medical and Inpatient Dermatology; Departments of Lerner College of Medicine of Case Western Dermatology and Pathology, Cleveland Clinic; Assistant Reserve University, Cleveland, OH Clinical Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH Cutaneous adverse effects of biologic medications ABSTRACT iologic therapy encompasses an expo- B nentially expanding arena of medicine. Biologic therapies have become widely used but often As the name implies, biologic therapies are de- cause cutaneous adverse effects. The authors discuss the rived from living organisms and consist largely cutaneous adverse effects of tumor necrosis factor (TNF) of proteins, sugars, and nucleic acids. A clas- alpha inhibitors, epidermal growth factor receptor (EGFR) sic example of an early biologic medication is inhibitors, small-molecule tyrosine kinase inhibitors insulin. These therapies have revolutionized (TKIs), and cell surface-targeted monoclonal antibodies, medicine and offer targeted therapy for an including how to manage these reactions -

Genomic Oncology: Moving Beyond the Tip of the Iceberg Jane De Lartigue, Phd
FeatureCommunity Report Genomic oncology: moving beyond the tip of the iceberg Jane de Lartigue, PhD istorically, cancer has been diagnosed and in patients with lung cancer, even the most efec- treated on the basis of the tissue of ori- tive targeted therapies can fail if used in the wrong Hgin. Te promise of personalized therapy, patient population.5,6 matched more precisely to an individual’s tumor, In recognition of this issue, the oncology feld has was ushered in with the development of molecularly developed molecular biomarkers that can predict targeted therapies, based on a greater understanding response, or lack thereof, to targeted therapy. Drugs of cancer as a genomic-driven disease. Here, we dis- are now commonly being evaluated in trials that cuss some of the evolution of genomic oncology, the select eligible patients on the basis of biomarker pos- inherent complexities and challenges, and how novel itivity, and a number of companion diagnostics have clinical trial designs are among the strategies being been codeveloped to assist in these eforts (Table 1). developed to address them and shape the future of Notable successes include the development of the personalized medicine in cancer. monoclonal antibody trastuzumab for patients with breast cancers that have human epidermal growth The evolution of genomic oncology factor receptor 2 (HER2) gene amplifcation or In the 15 years since the frst map of the human HER2 protein overexpression,7 and the small mol- genome emerged, genetics has become an inte- ecule BRAF kinase inhibitor -
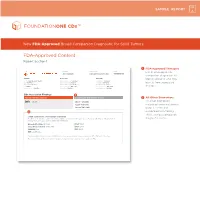
FDA-Approved Content Report Section 1
SAMPLE REPORT New FDA-Approved Broad Companion Diagnostic for Solid Tumors FDA-Approved Content Report Section 1 1 FDA-Approved Therapies PATIENT TUMOR TYPE TRF# List of FDA-approved Jane Sample Lung adenocarcinoma TRFXXXXXX companion diagnostics to PATIENT PHYSICIAN SPECIMEN identify patients who may DISEASE Lung adenocarcinoma ORDERING PHYSICIAN Not Given SPECIMEN SITE Not Given NAME Not Given MEDICAL FACILITY Not Given SPECIMEN ID Not Given benefi t from associated DATE OF BIRTH Not Given ADDITIONAL RECIPIENT Not Given SPECIMEN TYPE Not Given SEX Female MEDICAL FACILITY ID Not Given DATE OF COLLECTION Not Given therapies MEDICAL RECORD # Not Given PATHOLOGIST Not Given SPECIMEN RECEIVED Not Given CDx Associated Findings 1 GENOMIC FINDINGS DETECTED FDA-APPROVED THERAPEUTIC OPTIONS 2 All Other Biomarkers EGFR L858R Gilotrif® (Afatinib) All other biomarkers, Iressa® (Gefitinib) including tumor mutational Tarceva® (Erlotinib) burden (TMB) and 2 microsatellite instability (MSI), without companion OTHER ALTERATIONS & BIOMARKERS IDENTIFIED Results reported in this section are not prescriptive or conclusive for labeled use of any specific therapeutic product. See diagnostic claims professional services section for additional information. Microsatellite Status MS-Stable PTCH1 T416S Tumor Mutation Burden 11 Muts/Mb RBM10 Q494* CDKN2A/B loss TP53 R267P EGFR amplification § Refer to appendix for limitation statements related to detection of any copy number alterations, gene rearrangements, MSI or TMB result in this section. Please refer to appendix -

Summary Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 211675Orig1s000 SUMMARY REVIEW Cross Discipline Team Leader Review NDA 211675 Division Director Sununa1y Rinvoq (upadacitinib) for RA Office Director Sununary AbbVie , Inc. DHHS/FDA/CDER/ODEII/DPARP Cross-Discipline Team Leader Review Division Director Summary Office Director Summary Date July 11 , 201 9 Rachel Glaser, MD, Clinical Team Leader, DPARP From Sally Seymour, MD, Director, DP ARP Marv Thanh Hai, MD, Acting Director, ODEII Cross-Discipline Team Leader Review Subject Division Director Summaiy Office Director Summary NDA/BLA # and Supplement# 211675 Applicant AbbVie fuc Date of Submission December 18, 201 8 PDUFA Goal Date AUITTlSt 18, 201 9 Proprietary Name RINVOO Established or Proper Name Uoadacitinib Dosa2e Form(s) 15 mg extended release tablets Applicant Proposed (b)(4 Indication(s )/Population(s) Applicant Proposed Dosing 15 mg orally administered once daily Reoimen(s) Recommendation on Regulatory Approval Action Recommended Treatment of adults with moderately to severely active lndication(s)/Population(s) (if rheumatoid ai1hritis who have had an inadequate aoolicable) resoonse or intolerance to methotrexate Recommended Dosing 15 mg orally administered once daily Re2imen(s) (if aoolicable) CDER Cross Discipline Team Leader Review Template 1 Version date: October 10, 2017fo r all NDAs and BLAs Reference ID: 4478224 Cross Discipline Team Leader Review NDA 211675 Division Director Summary Rinvoq (upadacitinib) for RA Office Director Summary AbbVie, Inc. DHHS/FDA/CDER/ODEII/DPARP 1. Benefit-Risk Assessment Benefit-Risk Assessment Framework Rheumatoid arthritis (RA) is a serious medical condition that affects over 1.3 million Americans. RA is a chronic progressive disease that primarily affects the joints, but can involve other organs. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor