Embryology of Urogenital System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ROBO2 Restricts the Nephrogenic Field and Regulates Wolffian Duct–Nephrogenic Cord Separation
Developmental Biology 404 (2015) 88–102 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology ROBO2 restricts the nephrogenic field and regulates Wolffian duct–nephrogenic cord separation Elanor N. Wainwright 1, Dagmar Wilhelm 2, Alexander N. Combes 3, Melissa H. Little 4, Peter Koopman n Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia article info abstract Article history: ROBO2 plays a key role in regulating ureteric bud (UB) formation in the embryo, with mutations in Received 7 April 2015 humans and mice leading to supernumerary kidneys. Previous studies have established that the number Received in revised form and position of UB outgrowths is determined by the domain of metanephric mesenchymal Gdnf ex- 28 May 2015 pression, which is expanded anteriorly in Robo2 mouse mutants. To clarify how this phenotype arises, we Accepted 30 May 2015 used high-resolution 3D imaging to reveal an increase in the number of nephrogenic cord cells, leading Available online 23 June 2015 to extension of the metanephric mesenchyme field in Robo2-null mouse embryos. Ex vivo experiments Keywords: suggested a dependence of this effect on proliferative signals from the Wolffian duct. Loss of Robo2 Kidney resulted in a failure of the normal separation of the mesenchyme from the Wolffian duct/ureteric epi- Wolffian duct thelium, suggesting that aberrant juxtaposition of these two compartments in Robo2-null mice exposes Ureteric bud the mesenchyme to abnormally high levels of proliferative stimuli. Our data suggest a new model in Nephrogenic cord Mouse which SLIT-ROBO signalling acts not by attenuating Gdnf expression or activity, but instead by limiting epithelial/mesenchymal interactions in the nascent metanephros and restricting the extent of the ne- phrogenic field. -

Urinary System Intermediate Mesoderm
Urinary System Intermediate mesoderm lateral mesoderm: somite ectoderm neural NOTE: Intermediate mesoderm splanchnic groove somatic is situated between somites and lateral mesoderm (somatic and splanchnic mesoderm bordering the coelom). All mesoderm is derived from the primary mesen- intermediate mesoderm endoderm chyme that migrated through the notochord coelom (becomes urogenital ridge) primitive streak. Intermediate mesoderm (plus adjacent mesothelium lining the coelom) forms a urogenital ridge, which consists of a laterally-positioned nephrogenic cord (that forms kidneys & ureter) and a medially-positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Thus urinary & genital systems have a common embryonic origin; also, they share common ducts. NOTE: Urine production essentially requires an increased capillary surface area (glomeruli), epithelial tubules to collect plasma filtrate and extract desirable constituents, and a duct system to convey urine away from the body. Kidneys Bilateraly, three kid- mesonephric duct neys develop from the neph- metanephros pronephros rogenic cord. They develop mesonephric tubules chronologically in cranial- mesonephros caudal sequence, and are designated pro—, meso—, Nephrogenic Cord (left) and meta—, respectively. cloaca The pronephros and mesonephros develop similarly: the nephrogenic cord undergoes seg- mentation, segments become tubules, tubules drain into a duct & eventually tubules disintegrate. spinal ganglion 1] Pronephros—consists of (7-8) primitive tubules and a pronephric duct that grows caudally and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the neural tube mesonephric duct. (The pronephros is not functional, somite except in sheep.) notochord mesonephric NOTE tubule The mesonephros is the functional kidney for fish and am- aorta phibians. The metanephros is the functional kidney body of reptiles, birds, & mammals. -

The Derivatives of Three-Layered Embryo (Germ Layers)
HUMANHUMAN EMBRYOLOGYEMBRYOLOGY Department of Histology and Embryology Jilin University ChapterChapter 22 GeneralGeneral EmbryologyEmbryology FourthFourth week:week: TheThe derivativesderivatives ofof trilaminartrilaminar germgerm discdisc Dorsal side of the germ disc. At the beginning of the third week of development, the ectodermal germ layer has the shape of a disc that is broader in the cephalic than the caudal region. Cross section shows formation of trilaminar germ disc Primitive pit Drawing of a sagittal section through a 17-day embryo. The most cranial portion of the definitive notochord has formed. ectoderm Schematic view showing the definitive notochord. horizon =ectoderm hillside fields =neural plate mountain peaks =neural folds Cave sinks into mountain =neural tube valley =neural groove 7.1 Derivatives of the Ectodermal Germ Layer 1) Formation of neural tube Notochord induces the overlying ectoderm to thicken and form the neural plate. Cross section Animation of formation of neural plate When notochord is forming, primitive streak is shorten. At meanwhile, neural plate is induced to form cephalic to caudal end, following formation of notochord. By the end of 3rd week, neural folds and neural groove are formed. Neural folds fuse in the midline, beginning in cervical region and Cross section proceeding cranially and caudally. Neural tube is formed & invade into the embryo body. A. Dorsal view of a human embryo at approximately day 22. B. Dorsal view of a human embryo at approximately day 23. The nervous system is in connection with the amniotic cavity through the cranial and caudal neuropores. Cranial/anterior neuropore Neural fold heart Neural groove endoderm caudal/posterior neuropore A. -

Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E
1 Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E. Quaggin CHAPTER OUTLINE MAMMALIAN KIDNEY DEVELOPMENT, 2 MOLECULAR GENETICS OF MODEL SYSTEMS TO STUDY KIDNEY NEPHROGENESIS, 22 DEVELOPMENT, 8 GENETIC ANALYSIS OF MAMMALIAN KIDNEY DEVELOPMENT, 15 KEY POINTS • The development of the kidney relies on reciprocal signaling and inductive interactions between neighboring cells. • Epithelial cells that comprise the tubular structures of the kidney are derived from two distinct cell lineages: the ureteric epithelia lineage that branches and gives rise to collecting ducts and the nephrogenic mesenchyme lineage that undergoes mesenchyme to epithelial transition to form connecting tubules, distal tubules, the loop of Henle, proximal tubules, parietal epithelial cells, and podocytes. • Nephrogenesis and nephron endowment requires an epigenetically regulated balance between nephron progenitor self-renewal and epithelial differentiation. • The timing of incorporation of nephron progenitor cells into nascent nephrons predicts their positional identity within the highly patterned mature nephron. • Stromal cells and their derivatives coregulate ureteric branching morphogenesis, nephrogenesis, and vascular development. • Endothelial cells track the development of the ureteric epithelia and establish the renal vasculature through a combination of vasculogenic and angiogenic processes. • Collecting duct epithelia have an inherent plasticity enabling them to switch between principal and intercalated cell identities. MAMMALIAN KIDNEY DEVELOPMENT The filtration function of the kidneys is accomplished by basic units called nephrons (Fig. 1.1). Humans on average have 1 million nephrons per adult kidney but the range of ANATOMIC OVERVIEW OF THE 4 MAMMALIAN KIDNEY total nephrons is highly variable across human populations. Each mouse kidney may contain up to 12,000–16,000 nephrons The kidney is a sophisticated, highly vascularized organ that depending on the strain.5 This wide range in nephron number plays a central role in overall body homeostasis. -
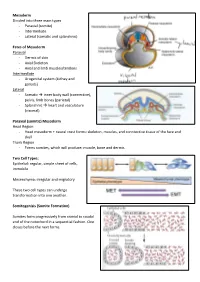
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -

Renal Development
RENAL DEVELOPMENT Jon Barasch M.D., Ph.D. Telephone: 305-1890 e-mail: [email protected] SUGGESTED READING: Larsen, 3rd edition, pp 265 (first three paragraphs) - 266, 268-276 and figure 10-10 LEARNING OBJECTIVES: You should be able to: 1. Describe the three kidneys that are produced during development and know what happens to each one. 2. Explain what is meant by ‘reciprocal induction’ and why it poses problems in interpreting experiments in developing kidney. 3. Describe the stages of nephron formation from the renal vesicle. 4. Discuss the regulators of mesenchymal to epithelial transition in the intermediate mesoderm and metanephric mesenchyme and name three molecules mediating conversion. 5. Describe branching morphogenesis and name the three patterns in the developing metanephros. 6. Discuss three key important ligands and their receptors. 7. Discuss the classification of congenital renal abnormalities that are associated with urological abnormalities and the possible underlying mechanisms for their association. SUMMARY: The urogenital system derives from mesenchymal cells by a process of conversion to epithelia. The development of the kidney relies on three mechanisms of epithelial morphogenesis. 1. Some newborn epithelia migrate extensively (Wolffian duct), 2. some undergo branching morphogenesis (ureteric bud) and 3. some produce highly segmented tubules (nephrons). GLOSSARY: Angiotensin II: your favorite vasoconstrictor and regulator of proximal tubule reclamation of NaCl and water by receptor type 1. Receptor type-2 modulates cell growth and seems to play a role in congenital abnormalities. Arcade: a tubule of ureteric bud that induces a few nephrons simultaneously. The nephrons join to a common drainage called a connecting tubule that feeds into the ureteric’s collecting duct. -

Kidney Development and Function in the Fetus
Kidney Development, - and Function in the Fetus Bob Caruthers, CST, PhD The kidneys produce urine, a blood filtrate, and regulate uri- can be seen in the fourth week. The pronephros is complete1 nary volume and composition. These regulatory activities regred by the start of the fifth week. The pronephros form involve balancing water and solute transport, conserving and regresses in a cranial-to-caudal sequence. No pronephric nutrients, eliminating waste products, and regulating acid and glomeruli (cluster of capillaries) have been observed, and no bases. The primary purpose of kidney function is to maintain a vesicles are associated with the pronephric duct. The stable environment in which cellular and tissue metabolic pronephros is, therefore, not active in urine producti0n.Y activity can proceed at an optimal level. The kidneys secrete the hormone renin, erythropoietin and 1.25-dihydroxy vita- MPSONEPHROS min D. Renin helps regulate blood pressure. Erythropoietin The mesonephros originates in the nephrogenic cord that is helps regulate erythrocyte production. 1,25-dihydroxy vitamin part of the intermediate mesoderm. Early mesonephric forma- D plays a role in calcium metabolism. This article will discuss tion is evident before the pronephros has completed its regre: the development of the kidney, and its role in the fetus."' sion. The mesonephros also degenerates in a cranial-to-cauda Since the kidneys are bilateral structures, development sequence. Some of the cranial structures are degenerating in involves both right and left kidneys. During fetal develop- the fifth week of fetal development while the caudal structun ment, three separate nephric structures develop in succession; are still differentiating. -

Imaging of Urachal Anomalies
Abdominal Radiology (2019) 44:3978–3989 https://doi.org/10.1007/s00261-019-02205-x SPECIAL SECTION : UROTHELIAL DISEASE Imaging of urachal anomalies Suryakala Buddha1 · Christine O. Menias2 · Venkata S. Katabathina1 Published online: 3 September 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Urachal anomalies are classifed into four types depending on the level of persistence of the embryonic urachal remnants between the urinary bladder and the umbilicus: patent urachus, umbilical–urachal sinus, urachal cyst, and vesico-urachal diverticulum. Due to the increasing use of cross-sectional imaging, urachal anomalies are frequently detected as incidental fndings. Imaging plays a pivotal role in the initial diagnosis, evaluation of complications, treatment follow-up, and long-term surveillance of patients with urachal anomalies. Diferent urachal anomalies demonstrate characteristic imaging features that aid in a timely diagnosis and guide treatment. A patent urachus is visualized as an elongated tubular structure between the umbilicus and the urinary bladder. While umbilical–urachal sinus appears as focal dilatation at the umbilical end of the urachal remnant, the vesico-urachal diverticulum presents as a focal outpouching of the urinary bladder at anterosuperior aspect. Urachal cysts are identifed as midline fuid-flled sacs most frequently located near the dome of the urinary bladder. Untreated urachal anomalies could progress into potential complications, including infection and malignancy. Knowledge regarding -

Unit 3 Embryo Questions
Unit 3 Embryology Clinically Oriented Anatomy (COA) Texas Tech University Health Sciences Center Created by Parker McCabe, Fall 2019 parker.mccabe@@uhsc.edu Solu%ons 1. B 11. A 21. D 2. C 12. B 22. D 3. C 13. E 23. D 4. B 14. D 24. A 5. E 15. C 25. D 6. C 16. B 26. B 7. D 17. E 27. C 8. B 18. A 9. C 19. C 10. D 20. B Digestive System 1. Which of the following structures develops as an outgrowth of the endodermal epithelium of the upper part of the duodenum? A. Stomach B. Pancreas C. Lung buds D. Trachea E. Esophagus Ques%on 1 A. Stomach- Foregut endoderm B. Pancreas- The pancreas, liver, and biliary apparatus all develop from outgrowths of the endodermal epithelium of the upper part of the duodenum. C. Lung buds- Foregut endoderm D. Trachea- Foregut endoderm E. Esophagus- Foregut endoderm 2. Where does the spleen originate and then end up after the rotation of abdominal organs during fetal development? A. Ventral mesentery à left side B. Ventral mesentery à right side C. Dorsal mesentery à left side D. Dorsal mesentery à right side E. It does not relocate Question 2 A. Ventral mesentery à left side B. Ventral mesentery à right side C. Dorsal mesentery à left side- The spleen and dorsal pancreas are embedded within the dorsal mesentery (greater omentum). After rotation, dorsal will go to the left side of the body and ventral will go to the right side of the body (except for the ventral pancreas). -

From Hatching Into Fetal Life in the P E in the P E in The
R.C. Chebel. 2011. Use of Applied Reproductive Technologies (FTAI, FTET) to Improve the Reproductive Efficiency in Dairy Cattle. Acta Scientiae Veterinariae. 39(Suppl 1): s203 - s221. Acta Scientiae Veterinariae, 2011. 39(Suppl 1): s203 - s221. ISSN 1679-9216 (Online) From Hatching into Fetal Life in the Pig Poul Hyttel, Kristian M. Kamstrup & Sara Hyldig ABSTRACT Background: Potential adverse effects of assisted reproductive technologies may have long term consequences on embryonic and fetal development. However, the complex developmental phases occurring after hatching from the zona pellucida are less studied than those occurring before hatching. The aim of the present review is to introduce the major post-hatching developmental features bringing the embryo form the blastocyst into fetal life in the pig. Review: In the pre-hatching mouse blastocyst, the pluripotency of the inner cell mass (ICM) is sustained through expression of OCT4 and NANOG. In the pre-hatching porcine blastocyst, a different and yet unresolved mechanism is operating as OCT4 is expressed in both the ICM and trophectoderm, and NANOG is not expressed at all. Around the time of hatching, OCT4 becomes confined to the ICM. In parallel, the ICM is divided into a ventral cell layer, destined to form the hypoblast, and a dorsal cell mass, destined to form the epiblast. The hypoblast gradually develops into a complete inner lining along the epiblast and the trophectoderm. Upon hatching (around Day 7-8 of gestation), the trophectoderm covering the developing epiblast (Rauber´s layer) is lost and the embryonic disc is formed by development of a cavity in the epiblast, which subsequently “unfolds” resulting the establishment of the disc. -

RENAL BLOCK Embryology Team Notes Development of Kidneys And
RENAL BLOCK Embryology Team Notes Development of kidneys and ureters Student Guide: 1- The notes, which are written by the team, are in Blue . 2- Everything written in Red is important. EMBRYOLOGICAL ORIGIN INTERMEDIATE MESODERM Differentiates into: Medial Lateral Nephrogenic ridge (cord) Gonadal ridge forms kidneys & ureters forms gonads (testes or ovaries) DEVELOPMENT OF KIDNEYS Three systems of kidney develops: 1-Pronephric system 2-Mesonephric system 3-Metanephric system Pronephric Mesonephric Metanephric system: system: system: • - appears at beginning of • - appears at end of 4th • - appears at 5th week in 4th week week pelvis • in cervical region • in thoracic & abdominal • - starts to function at 9th • - analogous to kidney of regions week fish • - analogous to kidney of • - formed of tubules & a amphibians duct • - formed of tubules & a • - not function in human duct • - disappears • - function temporarily • -The duct: In male: forms genital duct • - In both sexes: forms ureteric bud - The tubules disappear > ducts remain > its last part (ureteric bud) opens in the anterior aspect of cloaca (which is the bladder later) - In females it degenerates Ureteric Bud Metanephric Blastema 2 (mass) Forms: 3 METANEPHROS (PERMANENT KIDNEY) 2+3 Gives Collecting part of kidney Gives Excretory part of kidney 1 Cloaca 2 Ureter anlage 3 Metanephric blastema 2+3 Metanephros 4 Mesonephric duct (Wolffian duct) 5 Nephrogenic cord 4+5 Mesonephros COLLECTINGCOLLECTING PART PART: A- Ureteric B- Stalk of C- Branching D- Continuous bud elongates ureteric bud of renal pelvis branching & penetrates forms ureter gives 3 major gives straight metanephric & its cranial calices. then arched mass. end forms Branching of collecting renal pelvis. major calyces tubules gives minor calyces. -

Urinary System
Urinary System NOTE: Urine production requires an increased capillary surface area (glomeruli), epithelial tubules to collect plasma filtrate and extract desirable constituents, and a duct system to convey urine away from the body. Intermediate mesoderm: NOTE: lateral mesoderm: somite neural ectoderm All mesoderm is derived splanchnic groove from primary mesenchyme that somatic migrated through the primitive streak. Intermediate mesoderm is situated between somites and lateral mesoderm (somatic and intermediate mesoderm endoderm notochord coelom splanchnic mesoderm bordering (becomes urogenital ridge) the coelom). Intermediate mesoderm (including adjacent coelom mesothelium) forms a urogenital ridge, con- sisting of a laterally-positioned nephrogenic cord (that becomes kidneys & ureter) and a medially- positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Urinary & genital systems have a common embryonic origin; also, they share common ducts. Kidneys: mesonephric duct • three kidneys develop metanephros chronologically, in cranial- pronephros mesonephric tubules caudal sequence, from each bilateral nephrogenic cord mesonephros • the three kidneys are designated: Nephrogenic Cord (left) pro—, meso—, and meta—, respectively. cloaca • the pronephros and mesonephros have a similar development: — nephrogenic cord mesoderm undergoes segmentation, — segments become tubules that drain into a duct — eventually the tubules disintegrate. 1] Pronephros—consists of (7-8) primitive spinal ganglion tubules and a pronephric duct that grows caudal- ly and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the neural mesonephric duct. tube somite NOTE notochord • The pronephros is not functional, except in sheep. mesonephric • The mesonephros is functional in only some mammals tubule (related to placental layers). However, the mesonephros aorta becomes the functional kidney of adult fish & amphibians.