Disorders of the Bladder and Cloacal Anomaly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Guidelines on Paediatric Urology S
Guidelines on Paediatric Urology S. Tekgül (Chair), H.S. Dogan, E. Erdem (Guidelines Associate), P. Hoebeke, R. Ko˘cvara, J.M. Nijman (Vice-chair), C. Radmayr, M.S. Silay (Guidelines Associate), R. Stein, S. Undre (Guidelines Associate) European Society for Paediatric Urology © European Association of Urology 2015 TABLE OF CONTENTS PAGE 1. INTRODUCTION 7 1.1 Aim 7 1.2 Publication history 7 2. METHODS 8 3. THE GUIDELINE 8 3A PHIMOSIS 8 3A.1 Epidemiology, aetiology and pathophysiology 8 3A.2 Classification systems 8 3A.3 Diagnostic evaluation 8 3A.4 Disease management 8 3A.5 Follow-up 9 3A.6 Conclusions and recommendations on phimosis 9 3B CRYPTORCHIDISM 9 3B.1 Epidemiology, aetiology and pathophysiology 9 3B.2 Classification systems 9 3B.3 Diagnostic evaluation 10 3B.4 Disease management 10 3B.4.1 Medical therapy 10 3B.4.2 Surgery 10 3B.5 Follow-up 11 3B.6 Recommendations for cryptorchidism 11 3C HYDROCELE 12 3C.1 Epidemiology, aetiology and pathophysiology 12 3C.2 Diagnostic evaluation 12 3C.3 Disease management 12 3C.4 Recommendations for the management of hydrocele 12 3D ACUTE SCROTUM IN CHILDREN 13 3D.1 Epidemiology, aetiology and pathophysiology 13 3D.2 Diagnostic evaluation 13 3D.3 Disease management 14 3D.3.1 Epididymitis 14 3D.3.2 Testicular torsion 14 3D.3.3 Surgical treatment 14 3D.4 Follow-up 14 3D.4.1 Fertility 14 3D.4.2 Subfertility 14 3D.4.3 Androgen levels 15 3D.4.4 Testicular cancer 15 3D.5 Recommendations for the treatment of acute scrotum in children 15 3E HYPOSPADIAS 15 3E.1 Epidemiology, aetiology and pathophysiology -

Renal Agenesis, Renal Tubular Dysgenesis, and Polycystic Renal Diseases
Developmental & Structural Anomalies of the Genitourinary Tract DR. Alao MA Bowen University Teach Hosp Ogbomoso Picture test Introduction • Congenital Anomalies of the Kidney & Urinary Tract (CAKUT) Objectives • To review the embryogenesis of UGS and dysmorphogenesis of CAKUT • To describe the common CAKUT in children • To emphasize the role of imaging in the diagnosis of CAKUT Introduction •CAKUT refers to gross structural anomalies of the kidneys and or urinary tract present at birth. •Malformation of the renal parenchyma resulting in failure of normal nephron development as seen in renal dysplasia, renal agenesis, renal tubular dysgenesis, and polycystic renal diseases. Introduction •Abnormalities of embryonic migration of the kidneys as seen in renal ectopy (eg, pelvic kidney) and fusion anomalies, such as horseshoe kidney. •Abnormalities of the developing urinary collecting system as seen in duplicate collecting systems, posterior urethral valves, and ureteropelvic junction obstruction. Introduction •Prevalence is about 3-6 per 1000 births •CAKUT is one of the commonest anomalies found in human. •It constitute approximately 20 to 30 percent of all anomalies identified in the prenatal period •The presence of CAKUT in a child raises the chances of finding congenital anomalies of other organ-systems Why the interest in CAKUT? •Worldwide, CAKUT plays a causative role in 30 to 50 percent of cases of end-stage renal disease (ESRD), •The presence of CAKUT, especially ones affecting the bladder and lower tract adversely affects outcome of kidney graft after transplantation Why the interest in CAKUT? •They significantly predispose the children to UTI and urinary calculi •They may be the underlying basis for urinary incontinence Genes & Environment Interact to cause CAKUT? • Tens of different genes with role in nephrogenesis have been identified. -

1 This Document Was Originally Published in May 2012, and Last
MEDICAL STUDENT EDUCATION CURRICULUM This document was originally published in May 2012, and last amended in April 2020. This document will continue to be periodically updated to reflect the growing body of literature related to this topic. ADULT UTI Keywords: Urinary tract infection (UTI); cystitis; pyelonephritis; uropathogens; antibiotics. LEARNING OBJECTIVES: At the end of this unit, the student will be able to: 1. Outline the prevalence and socioeconomic impact of adult UTI 2. List the distinctions between urinary infection, contamination and colonization in diagnosing a UTI 3. List the important host and bacterial characteristics associated with a clinically important UTI 4. Name the most common gram negative and gram positive bacteria associated with adult UTI 5. Name the predominant organisms constituting normal perineal flora 6. List methods of urine collection and the advantages of each 7. Describe the different signs and symptoms associated with upper tract and lower tract adult UTIs 8. Describe and perform chemical and microscopic urinalysis, and its limits in the diagnosis of adult UTI 9. Name dominant pathogens or disease entities that need to be considered in the differential diagnosis of UTI 10. Describe the differences between complicated and uncomplicated adult UTI 11. List indications and use of imaging modalities in the diagnosis of adult UTI 12. Outline treatment principles of both complicated and uncomplicated adult UTIs including cystitis, pyelonephritis, epididymitis, and prostatitis Introduction Urinary tract infections are a troubling and increasingly dangerous condition treated by physicians from a number of specialties, including Urology. The landscape of diagnosis and management is changing as new resistance patterns emerge. -

Terminologia Embryologica
Terminologia Embryologica МЕЖДУНАРОДНЫЕ ТЕРМИНЫ ПО ЭМБРИОЛОГИИ ЧЕЛОВЕКА С ОФИЦИАЛЬНЫМ СПИСКОМ РУССКИХ ЭКВИВАЛЕНТОВ FEDERATIVE INTERNATIONAL PROGRAMME ON ANATOMICAL TERMINOLOGIES (FIPAT) РОССИЙСКАЯ ЭМБРИОЛОГИЧЕСКАЯ НОМЕНКЛАТУРНАЯ КОМИССИЯ Под редакцией акад. РАН Л.Л. Колесникова, проф. Н.Н. Шевлюка, проф. Л.М. Ерофеевой 2014 VI СОДЕРЖАНИЕ 44 Facies Лицо Face 46 Systema digestorium Пищеварительная система Alimentary system 47 Cavitas oris Ротовая полость Oral cavity 51 Pharynx Глотка Pharynx 52 Canalis digestorius; Canalis Пищеварительный канал Alimentary canal oesophagogastrointestinalis 52 Oesophagus Пищевод Oesophagus▲ 53 Gaster Желудок Stomach 54 Duodenum Двенадцатиперстная кишка Duodenum 55 Ansa umbilicalis intestini Пупочная кишечная петля Midgut loop; Umbilical intestinal loop 56 Jejunum et Ileum Тощая и подвздошная кишка Jejunum and Ileum 56 Intestinum crassum Толстая кишка Large intestine 58 Canalis analis Анальный канал Anal canal 58 Urenteron; Pars postcloacalis Постклоакальная часть кишки Postcloacal gut; intestini Tailgut; Endgut 59 Hepar Печень Liver 60 Ductus choledochus; Ductus Жёлчный проток Bile duct biliaris 61 Vesica biliaris et ductus Жёлчный пузырь и пузырный про- Gallbladder and cystic cysticus ток duct 61 Pancreas Поджелудочная железа Pancreas 63 Systema respiratorium Дыхательная система Respiratory system 63 Nasus Нос Nose 64 Pharynx Глотка, зев Pharynx 64 Formatio arboris respiratoriae Формирование дыхательной Formation of системы (бронхиального дерева) respiratory tree 67 Systema urinarium Мочевая система Urinary -

Urinary System Intermediate Mesoderm
Urinary System Intermediate mesoderm lateral mesoderm: somite ectoderm neural NOTE: Intermediate mesoderm splanchnic groove somatic is situated between somites and lateral mesoderm (somatic and splanchnic mesoderm bordering the coelom). All mesoderm is derived from the primary mesen- intermediate mesoderm endoderm chyme that migrated through the notochord coelom (becomes urogenital ridge) primitive streak. Intermediate mesoderm (plus adjacent mesothelium lining the coelom) forms a urogenital ridge, which consists of a laterally-positioned nephrogenic cord (that forms kidneys & ureter) and a medially-positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Thus urinary & genital systems have a common embryonic origin; also, they share common ducts. NOTE: Urine production essentially requires an increased capillary surface area (glomeruli), epithelial tubules to collect plasma filtrate and extract desirable constituents, and a duct system to convey urine away from the body. Kidneys Bilateraly, three kid- mesonephric duct neys develop from the neph- metanephros pronephros rogenic cord. They develop mesonephric tubules chronologically in cranial- mesonephros caudal sequence, and are designated pro—, meso—, Nephrogenic Cord (left) and meta—, respectively. cloaca The pronephros and mesonephros develop similarly: the nephrogenic cord undergoes seg- mentation, segments become tubules, tubules drain into a duct & eventually tubules disintegrate. spinal ganglion 1] Pronephros—consists of (7-8) primitive tubules and a pronephric duct that grows caudally and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the neural tube mesonephric duct. (The pronephros is not functional, somite except in sheep.) notochord mesonephric NOTE tubule The mesonephros is the functional kidney for fish and am- aorta phibians. The metanephros is the functional kidney body of reptiles, birds, & mammals. -

The Derivatives of Three-Layered Embryo (Germ Layers)
HUMANHUMAN EMBRYOLOGYEMBRYOLOGY Department of Histology and Embryology Jilin University ChapterChapter 22 GeneralGeneral EmbryologyEmbryology FourthFourth week:week: TheThe derivativesderivatives ofof trilaminartrilaminar germgerm discdisc Dorsal side of the germ disc. At the beginning of the third week of development, the ectodermal germ layer has the shape of a disc that is broader in the cephalic than the caudal region. Cross section shows formation of trilaminar germ disc Primitive pit Drawing of a sagittal section through a 17-day embryo. The most cranial portion of the definitive notochord has formed. ectoderm Schematic view showing the definitive notochord. horizon =ectoderm hillside fields =neural plate mountain peaks =neural folds Cave sinks into mountain =neural tube valley =neural groove 7.1 Derivatives of the Ectodermal Germ Layer 1) Formation of neural tube Notochord induces the overlying ectoderm to thicken and form the neural plate. Cross section Animation of formation of neural plate When notochord is forming, primitive streak is shorten. At meanwhile, neural plate is induced to form cephalic to caudal end, following formation of notochord. By the end of 3rd week, neural folds and neural groove are formed. Neural folds fuse in the midline, beginning in cervical region and Cross section proceeding cranially and caudally. Neural tube is formed & invade into the embryo body. A. Dorsal view of a human embryo at approximately day 22. B. Dorsal view of a human embryo at approximately day 23. The nervous system is in connection with the amniotic cavity through the cranial and caudal neuropores. Cranial/anterior neuropore Neural fold heart Neural groove endoderm caudal/posterior neuropore A. -

Imperforate Anus and Cloacal Malformations Marc A
C H A P T E R 3 5 Imperforate Anus and Cloacal Malformations Marc A. Levitt • Alberto Peña ‘Imperforate anus’ has been a well-known condition since component but were left with a persistent urogenital antiquity.1–3 For many centuries, physicians, as well as sinus.21,23 Additionally, most rectovestibular fistulas were individuals who practiced medicine, have tried to help erroneously called ‘rectovaginal fistula’.21 A rectoblad- these children by creating an orifice in the perineum. derneck fistula in males is the only true supralevator Many patients survived, most likely because they suffered malformation and occurs in about 10%.18 As it is the only from a type of defect that is now recognized as ‘low.’ malformation in males in which the rectum is unreach- Those with a ‘high’ defect did not survive. In 1835, able through a posterior sagittal incision, it requires an Amussat was the first to suture the rectal wall to the skin abdominal approach (via laparoscopy or a laparotomy) in edges which was the first actual anoplasty.2 Stephens addition to the perineal approach. made a significant contribution by performing the first Anorectal malformations represent a wide spectrum of anatomic studies in human specimens. In 1953, he pro- defects. The terms ‘low,’ ‘intermediate,’ and ‘high’ are arbi- posed an initial sacral approach followed by an abdomi- trary and not useful in current therapeutic or prognostic noperineal operation, if needed.4 The purpose of the terminology. A therapeutic and prognostically oriented sacral stage of this procedure was to preserve the pub- classification is depicted in Box 35-1.24 orectalis sling, considered a key factor in maintaining fecal incontinence. -

Laparoscopic Management of Urachal Cysts
Original Article Laparoscopic management of urachal cysts Salvatore Fabio Chiarenza, Cosimo Bleve Department of Pediatric Surgery and Pediatric Minimally Invasive Surgery and New Technologies, San Bortolo Hospital, Vicenza, Italy Contributions: (I) Conception and design: All authors; (II) Administrative support: All Authors; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Dr. Salvatore Fabio Chiarenza. Director of Department of Pediatric Surgery and Pediatric Minimally Invasive Surgery and New Technologies, San Bortolo Hospital, Vicenza, Italy. Email: [email protected]; Dr. Cosimo Bleve, MD. Department of Pediatric Surgery and Pediatric Minimally Invasive Surgery and New Technologies San Bortolo Hospital, Vicenza, Italy. Email: [email protected]. Background: The urachus and the urachal remnants represent a failure in the obliteration of the allantois at birth that connects the bladder to the umbilicus. After birth it obliterates and presents as the midline umbilical ligament. Urachal cyst are the most common urachal anomaly in the pediatric population. The traditional surgical approach is a semicircular infraumbilical incision or a lower midline laparotomy. Methods: In a 10 years period at Pediatric Surgery Department of Vicenza 16 children were diagnosed with urachal anomalies presenting as abdominal or urinary symptoms. Eight underwent open excision; eight were treated by laparoscopic surgery. The average age was 5.5 years (range, 4 months–13 years) in open group and 10 years (range, 1 month–18 years) in laparoscopic group. Results: Mean operative time was 63 minutes (range, 35–105 minutes) in open group, 50 minutes (range, 35–90 minutes) in laparoscopic group. -

Irish Rare Kidney Disease Network (IRKDN)
Irish Rare kidney Disease Network (IRKDN) Others Cork University Mater, Waterford University Dr Liam Plant Hospital Galway Dr Abernathy University Hospital Renal imaging Dr M Morrin Prof Griffin Temple St and Crumlin Beaumont Hospital CHILDRENS Hospital Tallaght St Vincents Dr Atiff Awann Rare Kidney Disease Clinic Hospital University Hospital Prof Peter Conlon Dr Lavin Prof Dr Holian Little Renal pathology Lab Limerick University Dr Dorman and Hospital Dr Doyle Dr Casserly Patient Renal Council Genetics St James Laboratory Hospital RCSI Dr Griffin Prof Cavaller MISION Provision of care to patients with Rare Kidney Disease based on best available medical evidence through collaboration within Ireland and Europe Making available clinical trials for rare kidney disease to Irish patients where available Collaboration with other centres in Europe treating rare kidney disease Education of Irish nephrologists on rare Kidney Disease. Ensuring a seamless transition of children from children’s hospital with rare kidney disease to adult centres with sharing of knowledge of rare paediatric kidney disease with adult centres The provision of precise molecular diagnosis of patients with rare kidney disease The provision of therapeutic plan based on understanding of molecular diagnosis where available Development of rare disease specific registries within national renal It platform ( Emed) Structure Beaumont Hospital will act as National rare Kidney Disease Coordinating centre working in conjunction with a network of Renal unit across the country -

Early Vaginal Replacement in Cloacal Malformation
Pediatric Surgery International (2019) 35:263–269 https://doi.org/10.1007/s00383-018-4407-1 ORIGINAL ARTICLE Early vaginal replacement in cloacal malformation Shilpa Sharma1 · Devendra K. Gupta1 Accepted: 18 October 2018 / Published online: 30 October 2018 © Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract Purpose We assessed the surgical outcome of cloacal malformation (CM) with emphasis on need and timing of vaginal replacement. Methods An ambispective study of CM was carried out including prospective cases from April 2014 to December 2017 and retrospective cases that came for routine follow-up. Early vaginal replacement was defined as that done at time of bowel pull through. Surgical procedures and associated complications were noted. The current state of urinary continence, faecal continence and renal functions was assessed. Results 18 patients with CM were studied with median age at presentation of 5 days (1 day–4 years). 18;3;2 babies underwent colostomy; vaginostomy; vesicostomy. All patients underwent posterior sagittal anorectovaginourethroplasty (PSARVUP)/ Pull through at a median age of 13 (4–46) months. Ten patients had long common channel length (> 3 cm). Six patients underwent early vaginal replacement at a median age of 14 (7–25) months with ileum; sigmoid colon; vaginal switch; hemirectum in 2;2;1;1. Three with long common channel who underwent only PSARVUP had inadequate introitus at puberty. Complications included anal mucosal prolapse, urethrovaginal fistula, perineal wound dehiscence, pyometrocolpos, blad- der injury and pelvic abscess. Persistent vesicoureteric reflux remained in 8. 5;2 patients had urinary; faecal incontinence. 2 patients of uterus didelphys are having menorrhagia. -
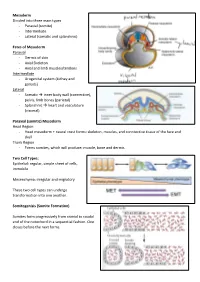
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop.