Defects of Urogenital Development in Mice Lacking Emx2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ROBO2 Restricts the Nephrogenic Field and Regulates Wolffian Duct–Nephrogenic Cord Separation
Developmental Biology 404 (2015) 88–102 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology ROBO2 restricts the nephrogenic field and regulates Wolffian duct–nephrogenic cord separation Elanor N. Wainwright 1, Dagmar Wilhelm 2, Alexander N. Combes 3, Melissa H. Little 4, Peter Koopman n Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia article info abstract Article history: ROBO2 plays a key role in regulating ureteric bud (UB) formation in the embryo, with mutations in Received 7 April 2015 humans and mice leading to supernumerary kidneys. Previous studies have established that the number Received in revised form and position of UB outgrowths is determined by the domain of metanephric mesenchymal Gdnf ex- 28 May 2015 pression, which is expanded anteriorly in Robo2 mouse mutants. To clarify how this phenotype arises, we Accepted 30 May 2015 used high-resolution 3D imaging to reveal an increase in the number of nephrogenic cord cells, leading Available online 23 June 2015 to extension of the metanephric mesenchyme field in Robo2-null mouse embryos. Ex vivo experiments Keywords: suggested a dependence of this effect on proliferative signals from the Wolffian duct. Loss of Robo2 Kidney resulted in a failure of the normal separation of the mesenchyme from the Wolffian duct/ureteric epi- Wolffian duct thelium, suggesting that aberrant juxtaposition of these two compartments in Robo2-null mice exposes Ureteric bud the mesenchyme to abnormally high levels of proliferative stimuli. Our data suggest a new model in Nephrogenic cord Mouse which SLIT-ROBO signalling acts not by attenuating Gdnf expression or activity, but instead by limiting epithelial/mesenchymal interactions in the nascent metanephros and restricting the extent of the ne- phrogenic field. -

Urinary System Intermediate Mesoderm
Urinary System Intermediate mesoderm lateral mesoderm: somite ectoderm neural NOTE: Intermediate mesoderm splanchnic groove somatic is situated between somites and lateral mesoderm (somatic and splanchnic mesoderm bordering the coelom). All mesoderm is derived from the primary mesen- intermediate mesoderm endoderm chyme that migrated through the notochord coelom (becomes urogenital ridge) primitive streak. Intermediate mesoderm (plus adjacent mesothelium lining the coelom) forms a urogenital ridge, which consists of a laterally-positioned nephrogenic cord (that forms kidneys & ureter) and a medially-positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Thus urinary & genital systems have a common embryonic origin; also, they share common ducts. NOTE: Urine production essentially requires an increased capillary surface area (glomeruli), epithelial tubules to collect plasma filtrate and extract desirable constituents, and a duct system to convey urine away from the body. Kidneys Bilateraly, three kid- mesonephric duct neys develop from the neph- metanephros pronephros rogenic cord. They develop mesonephric tubules chronologically in cranial- mesonephros caudal sequence, and are designated pro—, meso—, Nephrogenic Cord (left) and meta—, respectively. cloaca The pronephros and mesonephros develop similarly: the nephrogenic cord undergoes seg- mentation, segments become tubules, tubules drain into a duct & eventually tubules disintegrate. spinal ganglion 1] Pronephros—consists of (7-8) primitive tubules and a pronephric duct that grows caudally and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the neural tube mesonephric duct. (The pronephros is not functional, somite except in sheep.) notochord mesonephric NOTE tubule The mesonephros is the functional kidney for fish and am- aorta phibians. The metanephros is the functional kidney body of reptiles, birds, & mammals. -

Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E
1 Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E. Quaggin CHAPTER OUTLINE MAMMALIAN KIDNEY DEVELOPMENT, 2 MOLECULAR GENETICS OF MODEL SYSTEMS TO STUDY KIDNEY NEPHROGENESIS, 22 DEVELOPMENT, 8 GENETIC ANALYSIS OF MAMMALIAN KIDNEY DEVELOPMENT, 15 KEY POINTS • The development of the kidney relies on reciprocal signaling and inductive interactions between neighboring cells. • Epithelial cells that comprise the tubular structures of the kidney are derived from two distinct cell lineages: the ureteric epithelia lineage that branches and gives rise to collecting ducts and the nephrogenic mesenchyme lineage that undergoes mesenchyme to epithelial transition to form connecting tubules, distal tubules, the loop of Henle, proximal tubules, parietal epithelial cells, and podocytes. • Nephrogenesis and nephron endowment requires an epigenetically regulated balance between nephron progenitor self-renewal and epithelial differentiation. • The timing of incorporation of nephron progenitor cells into nascent nephrons predicts their positional identity within the highly patterned mature nephron. • Stromal cells and their derivatives coregulate ureteric branching morphogenesis, nephrogenesis, and vascular development. • Endothelial cells track the development of the ureteric epithelia and establish the renal vasculature through a combination of vasculogenic and angiogenic processes. • Collecting duct epithelia have an inherent plasticity enabling them to switch between principal and intercalated cell identities. MAMMALIAN KIDNEY DEVELOPMENT The filtration function of the kidneys is accomplished by basic units called nephrons (Fig. 1.1). Humans on average have 1 million nephrons per adult kidney but the range of ANATOMIC OVERVIEW OF THE 4 MAMMALIAN KIDNEY total nephrons is highly variable across human populations. Each mouse kidney may contain up to 12,000–16,000 nephrons The kidney is a sophisticated, highly vascularized organ that depending on the strain.5 This wide range in nephron number plays a central role in overall body homeostasis. -

Renal Development
RENAL DEVELOPMENT Jon Barasch M.D., Ph.D. Telephone: 305-1890 e-mail: [email protected] SUGGESTED READING: Larsen, 3rd edition, pp 265 (first three paragraphs) - 266, 268-276 and figure 10-10 LEARNING OBJECTIVES: You should be able to: 1. Describe the three kidneys that are produced during development and know what happens to each one. 2. Explain what is meant by ‘reciprocal induction’ and why it poses problems in interpreting experiments in developing kidney. 3. Describe the stages of nephron formation from the renal vesicle. 4. Discuss the regulators of mesenchymal to epithelial transition in the intermediate mesoderm and metanephric mesenchyme and name three molecules mediating conversion. 5. Describe branching morphogenesis and name the three patterns in the developing metanephros. 6. Discuss three key important ligands and their receptors. 7. Discuss the classification of congenital renal abnormalities that are associated with urological abnormalities and the possible underlying mechanisms for their association. SUMMARY: The urogenital system derives from mesenchymal cells by a process of conversion to epithelia. The development of the kidney relies on three mechanisms of epithelial morphogenesis. 1. Some newborn epithelia migrate extensively (Wolffian duct), 2. some undergo branching morphogenesis (ureteric bud) and 3. some produce highly segmented tubules (nephrons). GLOSSARY: Angiotensin II: your favorite vasoconstrictor and regulator of proximal tubule reclamation of NaCl and water by receptor type 1. Receptor type-2 modulates cell growth and seems to play a role in congenital abnormalities. Arcade: a tubule of ureteric bud that induces a few nephrons simultaneously. The nephrons join to a common drainage called a connecting tubule that feeds into the ureteric’s collecting duct. -
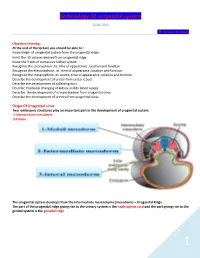
Embryology of Urogenital System
Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Objective learning : At the end of the lecture you should be able to : Know Origin of urogenital system from the urogenital ridge Enlist the Structures derived from urogenital ridge Know the 3 sets of successive kidney system Recognize the pronephron ,its time of appearance ,location and function Recognize the Mesonephron, its time of appearance ,location and function Recognize the metanephron ,its source, time of appearance ,location and function Describe the development of ureter from ureteric bud Describe the development of collecting duct Describe Positional changing of kidney and its blood supply Describe the development of urinary bladder from urogenital sinus Describe the development of urethra from urogenital sinus Origin Of Urogenital sinus Two embryonic structures play an important part in the development of urogenital system: 1-Intermediate mesoderm 2-Cloaca The urogenital system develops from the intermediate mesenchyme (mesoderm) – Urogenital Ridge. The part of the urogenital ridge giving rise to the urinary system is the nephrogenic cord and the part giving rise to the genital system is the gonadal ridge. 1 Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Development of urinary system: 3 sets of successive kidneys : Pronephron: These bilateral structures appear early in the fourth week. Segmented division of intermediate mesoderm form a few cell clusters and 5-7 pairs pronephric tubules in the cervical region. One end of the tubules opened at coelomic cavity and the other end opened in to pronephric duct 2 Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Function : The tubules transmits the waste product from coelomic cavity to the pronephric duct that runs caudally and open into the Cloaca . -

Kidney Development and Function in the Fetus
Kidney Development, - and Function in the Fetus Bob Caruthers, CST, PhD The kidneys produce urine, a blood filtrate, and regulate uri- can be seen in the fourth week. The pronephros is complete1 nary volume and composition. These regulatory activities regred by the start of the fifth week. The pronephros form involve balancing water and solute transport, conserving and regresses in a cranial-to-caudal sequence. No pronephric nutrients, eliminating waste products, and regulating acid and glomeruli (cluster of capillaries) have been observed, and no bases. The primary purpose of kidney function is to maintain a vesicles are associated with the pronephric duct. The stable environment in which cellular and tissue metabolic pronephros is, therefore, not active in urine producti0n.Y activity can proceed at an optimal level. The kidneys secrete the hormone renin, erythropoietin and 1.25-dihydroxy vita- MPSONEPHROS min D. Renin helps regulate blood pressure. Erythropoietin The mesonephros originates in the nephrogenic cord that is helps regulate erythrocyte production. 1,25-dihydroxy vitamin part of the intermediate mesoderm. Early mesonephric forma- D plays a role in calcium metabolism. This article will discuss tion is evident before the pronephros has completed its regre: the development of the kidney, and its role in the fetus."' sion. The mesonephros also degenerates in a cranial-to-cauda Since the kidneys are bilateral structures, development sequence. Some of the cranial structures are degenerating in involves both right and left kidneys. During fetal develop- the fifth week of fetal development while the caudal structun ment, three separate nephric structures develop in succession; are still differentiating. -

RENAL BLOCK Embryology Team Notes Development of Kidneys And
RENAL BLOCK Embryology Team Notes Development of kidneys and ureters Student Guide: 1- The notes, which are written by the team, are in Blue . 2- Everything written in Red is important. EMBRYOLOGICAL ORIGIN INTERMEDIATE MESODERM Differentiates into: Medial Lateral Nephrogenic ridge (cord) Gonadal ridge forms kidneys & ureters forms gonads (testes or ovaries) DEVELOPMENT OF KIDNEYS Three systems of kidney develops: 1-Pronephric system 2-Mesonephric system 3-Metanephric system Pronephric Mesonephric Metanephric system: system: system: • - appears at beginning of • - appears at end of 4th • - appears at 5th week in 4th week week pelvis • in cervical region • in thoracic & abdominal • - starts to function at 9th • - analogous to kidney of regions week fish • - analogous to kidney of • - formed of tubules & a amphibians duct • - formed of tubules & a • - not function in human duct • - disappears • - function temporarily • -The duct: In male: forms genital duct • - In both sexes: forms ureteric bud - The tubules disappear > ducts remain > its last part (ureteric bud) opens in the anterior aspect of cloaca (which is the bladder later) - In females it degenerates Ureteric Bud Metanephric Blastema 2 (mass) Forms: 3 METANEPHROS (PERMANENT KIDNEY) 2+3 Gives Collecting part of kidney Gives Excretory part of kidney 1 Cloaca 2 Ureter anlage 3 Metanephric blastema 2+3 Metanephros 4 Mesonephric duct (Wolffian duct) 5 Nephrogenic cord 4+5 Mesonephros COLLECTINGCOLLECTING PART PART: A- Ureteric B- Stalk of C- Branching D- Continuous bud elongates ureteric bud of renal pelvis branching & penetrates forms ureter gives 3 major gives straight metanephric & its cranial calices. then arched mass. end forms Branching of collecting renal pelvis. major calyces tubules gives minor calyces. -

Urinary System
Urinary System NOTE: Urine production requires an increased capillary surface area (glomeruli), epithelial tubules to collect plasma filtrate and extract desirable constituents, and a duct system to convey urine away from the body. Intermediate mesoderm: NOTE: lateral mesoderm: somite neural ectoderm All mesoderm is derived splanchnic groove from primary mesenchyme that somatic migrated through the primitive streak. Intermediate mesoderm is situated between somites and lateral mesoderm (somatic and intermediate mesoderm endoderm notochord coelom splanchnic mesoderm bordering (becomes urogenital ridge) the coelom). Intermediate mesoderm (including adjacent coelom mesothelium) forms a urogenital ridge, con- sisting of a laterally-positioned nephrogenic cord (that becomes kidneys & ureter) and a medially- positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Urinary & genital systems have a common embryonic origin; also, they share common ducts. Kidneys: mesonephric duct • three kidneys develop metanephros chronologically, in cranial- pronephros mesonephric tubules caudal sequence, from each bilateral nephrogenic cord mesonephros • the three kidneys are designated: Nephrogenic Cord (left) pro—, meso—, and meta—, respectively. cloaca • the pronephros and mesonephros have a similar development: — nephrogenic cord mesoderm undergoes segmentation, — segments become tubules that drain into a duct — eventually the tubules disintegrate. 1] Pronephros—consists of (7-8) primitive spinal ganglion tubules and a pronephric duct that grows caudal- ly and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the neural mesonephric duct. tube somite NOTE notochord • The pronephros is not functional, except in sheep. mesonephric • The mesonephros is functional in only some mammals tubule (related to placental layers). However, the mesonephros aorta becomes the functional kidney of adult fish & amphibians. -

A Coordinated Progression of Progenitor Cell States Initiates Urinary Tract Development
ARTICLE https://doi.org/10.1038/s41467-021-22931-5 OPEN A coordinated progression of progenitor cell states initiates urinary tract development Oraly Sanchez-Ferras 1, Alain Pacis1,2, Maria Sotiropoulou3, Yuhong Zhang1, Yu Chang Wang3, ✉ Mathieu Bourgey2,3, Guillaume Bourque 2,3, Jiannis Ragoussis 3,4 & Maxime Bouchard 1 The kidney and upper urinary tract develop through reciprocal interactions between the ureteric bud and the surrounding mesenchyme. Ureteric bud branching forms the arborized 1234567890():,; collecting duct system of the kidney, while ureteric tips promote nephron formation from dedicated progenitor cells. While nephron progenitor cells are relatively well characterized, the origin of ureteric bud progenitors has received little attention so far. It is well established that the ureteric bud is induced from the nephric duct, an epithelial duct derived from the intermediate mesoderm of the embryo. However, the cell state transitions underlying the progression from intermediate mesoderm to nephric duct and ureteric bud remain unknown. Here we show that nephric duct morphogenesis results from the coordinated organization of four major progenitor cell populations. Using single cell RNA-seq and Cluster RNA-seq, we show that these progenitors emerge in time and space according to a stereotypical pattern. We identify the transcription factors Tfap2a/b and Gata3 as critical coordinators of this progenitor cell progression. This study provides a better understanding of the cellular origin of the renal collecting duct system and associated urinary tract developmental diseases, which may inform guided differentiation of functional kidney tissue. 1 Goodman Cancer Research Centre and Department of Biochemistry, McGill University, Montreal, QC, Canada. 2 Canadian Centre for Computational Genomics, McGill University, Montréal, QC, Canada. -

Urogenital System - Development
Urogenital system - Development Aleš Hampl Urogenital system – Overall picture cloaca Urogenital system – Reminder Urogenital system – Intermediate mesoderm Urogenital system – Early forms of kidneys - Pronephros Recapitulation of three stages of evolution of kidneys in a cranial to caudal sequence: • pronephros • mesonephros • metanephros pronephros primary nephric duct Nephrotomes Nephric duct Nephrotomes • at about day 22 in cervical part of nephrogenic cord • 7 to 10 groups of epithelial cells • connect to primary nephric duct • non-functional • disappear by day 28 Urogenital system – Early forms of kidneys - Pronephros Mouse D9 – equivalent to human D27 The lumen of each nephrotome opens into the primary nephric duct as well as into the body cavity. Glomeruli form as small vessels extend from the dorsal aortae. Urogenital system – Early forms of kidneys - Mesonephros Mesonephros • caudal continuation of nephrogenic cord • thoracolumbar region • unsegmented intermediate mesoderm • mesonephric ducts (paired) – Wolffian ducts • mesonephric tubuli – open individually into m. duct • 36 to 40 m. tubuli in total (on one side) • some filtration – mesonephric unit • mesonephros is most prominent when metanephros start to shape • then they diasappear fast • mesonephric ducts persist in males Urogenital system – Mesonephros - Another view Urogenital system – Definitive kidneys - Metanephros Develop since week 5 Ureteric bud = metanefric diverticulum + Metanephrogenic blastema (mesenchyme) Branching and Elongation 14 to 15 x Urogenital system -
Signaling During Kidney Development
Cells 2015, 4, 112-132; doi:10.3390/cells4020112 OPEN ACCESS cells ISSN 2073-4409 www.mdpi.com/journal/cells Review Signaling during Kidney Development Mirja Krause 1, Aleksandra Rak-Raszewska 1, Ilkka Pietilä 1, Susan E. Quaggin 2 and Seppo Vainio 1,* 1 Faculty of Biochemistry and Molecular Medicine, Biocenter Oulu, Oulu University, 90014 Oulu, Finland; E-Mails: [email protected] (M.K.); [email protected] (A.R.R.); [email protected] (I.P.) 2 Northwestern University, Feinberg School of Medicine, Chicago, IL 60611, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +358-407-470939. Academic Editor: Christoph Englert Received: 28 February 2015 / Accepted: 30 March 2015 / Published: 10 April 2015 Abstract: The kidney plays an essential role during excretion of metabolic waste products, maintenance of key homeostasis components such as ion concentrations and hormone levels. It influences the blood pressure, composition and volume. The kidney tubule system is composed of two distinct cell populations: the nephrons forming the filtering units and the collecting duct system derived from the ureteric bud. Nephrons are composed of glomeruli that filter the blood to the Bowman’s capsule and tubular structures that reabsorb and concentrate primary urine. The collecting duct is a Wolffian duct-derived epithelial tube that concentrates and collects urine and transfers it via the renal pelvis into the bladder. The mammalian kidney function depends on the coordinated development of specific cell types within a precise architectural framework. Due to the availability of modern analysis techniques, the kidney has become a model organ defining the paradigm to study organogenesis. -

Genmedicine/Lect6 Podzimní Semestr
Lecture 6 General medicine_3rd semester DEVELOPMENT OF URINARY SYSTEM AND OVERVIEW OF ITS CONGENITAL MALFORMATION DEVELOPMENT OF REPRODUCTIVE SYSTEM: INDIFFERENT STAGE DEVELOPMENT OF INTERNAL AND EXTERNAL SEXUAL ORGANS OVERVIEW OF MOST IMPORTANT CONGENITAL MALFORMATIONS Introduction the urinary system and internal sexual organs develop from the intermediate mesoderm or nephrotomes a part of the third germ layer interposed between the axial mesoderm or somites and lateral mesoderm the intermediate mesoderm extends along the entire length of the dorsal body wall of the embryo it soon loses connection with somites and fuses to form the nephrogenic cords on each side of the primitive aorta the cords rapidly grow, become larger and produce bilateral longitudinal bulges called the urogenital ridges a medial side of each ridge is then separated from the surrounding and is called as the gonadal ridge (see the next chapter) DEVELOPMENT OF KIDNEYS, URETERS, BLADDER, AND URETHRA in the human, three sets of excretory organs develop: the pronephros (-oi) - "forkidney" - is rudimentary and nonfunctional; forkidney is analogous to the kidney of some primitive fishes, the mesonephros (-oi) -" midkidney" - is analogous to kidney of fishes and larval stages of amphibia amphibians; in human embryos, midkidney is in function for a short time and then undergoes involution the metanephros (-oi) - "hindkidney" or permanent kidney, it begins to produce urine in fetuses aged 11 to 13 weeks Pronephros (oi) occurs early in the fourth week in the cervical region