Genmedicine/Lect6 Podzimní Semestr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Structure of Pronephros and Development of Mesonephric Kidney in Larvae of Russian Sturgeon, Acipenser Gueldenstaedtii Brandt (Acipenseridae)
Zoologica5 PRONEPHROS Poloniae-AND (2012)-MESONEPHRIC 57/1-4: 5-20-KIDNEY-IN-LARVAE-OF-A.-GUELDENSTAEDTII 5 DOI: 10.2478/v10049-012-0001-6 STRUCTURE OF PRONEPHROS AND DEVELOPMENT OF MESONEPHRIC KIDNEY IN LARVAE OF RUSSIAN STURGEON, ACIPENSER GUELDENSTAEDTII BRANDT (ACIPENSERIDAE) L.S. KRAYUSHKINA*1, A.A. GERASIMOV1, A.A. KIRSANOV1, M.V. MOSYAGINA1, A. OGORZA£EK2 1Department of Ichthyology and Hydrobiology, St. Petersburg State University, 16-th Line 29, 199178, St. Petersburg, Russia, [email protected] 2 Department of Animal Developmental Biology, Zoological Institute, University of Wroclaw, Sienkiewicza 21, 50-335 Wroclaw, Poland. *Corresponding author Abstract. The structure of the pronephros and development of mesonephric kidney in Russian sturgeon larvae, Acipenser gueldenstaedtii Brandt at different stages of early postembryonic development (from hatching to 14 days), were studied with histological and electronic microscopy methods. The larval pronephros is represented by the system of bilaterally located pronephric tubules with ciliated nephrostomes and funnels and exog- enous single glomus, which is not integrated directly into pronephric tubules and located in the pronephric chamber. The glomus is positioned below the dorsal aorta and vascular- ized by its capillaries. The glomus has the same features of the thin structure that are typical of and necessary for the function of a filtering organ. The structure of the prone- phros in acipenserids is discussed and compared with teleosts and amphibians. Histogen- esis of the mesonephric kidney is observed during the period of pronephros functioning; it is complete by the time the larvae transfer to exogenous feeding. At this moment, the pronephros undergoes significant structural degradation. -

Development of the Female Reproductive System
Development of the female Reproductive System Dr. Susheela Rani Genital System •Gonads •Internal genitals •External genitals Determining sex – chronology of events •Determined Genetic sex at fertilization Gonadal sex •6th week Phenotypic sex •Differentiation of Behavioural Psyche - Preoptic and Median region Sex of Hypothalamus Genetic Sex Genetic sex of an embryo is determined at the time of fertilization, depending on whether the spermatocyte carries an X or a Y chromosome. The ‘Master’ Gene that determines Gender • SRY (Sex determining Region Y gene) • Has a testis-determining effect on the indifferent gonads. • Small gene (a single exon) • Localized on the shorter arm of the Y chromosome (Yp) • Gets expressed in the gonadal cells • Controls a whole number of further genes on the autosomes as well as on the X chromosome. • Causes development of Testes • Pseudo autosomal regions PAR1 and PAR 2 – Yellow • Heterochromatin – redundant DNA sequences – Pink • SRY – Region for Sex Determining Gene- Dark red • ZFY , Y linked Zinc Finger Protein – Orange • Spermatogenesis Genes in long arm – Azoospermia factor AZF • Telomeres – green • Centromeres - Blue It is not the number of gonosomes that is decisive for the gender, but rather the presence or absence of the Y-chromosome Aneuploidy and Euploidy of Gonosomes Karyotype Phenotypic Gonad Syndrome Fate Gender 45, XO Female Ovaries Turner’s Atrophy of Ovaries in the fetus 45, YO ------ ----- ----- Absence of X chromosome is lethal 46, XX Female Ovaries Normal Normal Development Woman 47, XXX Female -

ROBO2 Restricts the Nephrogenic Field and Regulates Wolffian Duct–Nephrogenic Cord Separation
Developmental Biology 404 (2015) 88–102 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology ROBO2 restricts the nephrogenic field and regulates Wolffian duct–nephrogenic cord separation Elanor N. Wainwright 1, Dagmar Wilhelm 2, Alexander N. Combes 3, Melissa H. Little 4, Peter Koopman n Institute for Molecular Bioscience, The University of Queensland, Brisbane, QLD 4072, Australia article info abstract Article history: ROBO2 plays a key role in regulating ureteric bud (UB) formation in the embryo, with mutations in Received 7 April 2015 humans and mice leading to supernumerary kidneys. Previous studies have established that the number Received in revised form and position of UB outgrowths is determined by the domain of metanephric mesenchymal Gdnf ex- 28 May 2015 pression, which is expanded anteriorly in Robo2 mouse mutants. To clarify how this phenotype arises, we Accepted 30 May 2015 used high-resolution 3D imaging to reveal an increase in the number of nephrogenic cord cells, leading Available online 23 June 2015 to extension of the metanephric mesenchyme field in Robo2-null mouse embryos. Ex vivo experiments Keywords: suggested a dependence of this effect on proliferative signals from the Wolffian duct. Loss of Robo2 Kidney resulted in a failure of the normal separation of the mesenchyme from the Wolffian duct/ureteric epi- Wolffian duct thelium, suggesting that aberrant juxtaposition of these two compartments in Robo2-null mice exposes Ureteric bud the mesenchyme to abnormally high levels of proliferative stimuli. Our data suggest a new model in Nephrogenic cord Mouse which SLIT-ROBO signalling acts not by attenuating Gdnf expression or activity, but instead by limiting epithelial/mesenchymal interactions in the nascent metanephros and restricting the extent of the ne- phrogenic field. -

Urinary System Intermediate Mesoderm
Urinary System Intermediate mesoderm lateral mesoderm: somite ectoderm neural NOTE: Intermediate mesoderm splanchnic groove somatic is situated between somites and lateral mesoderm (somatic and splanchnic mesoderm bordering the coelom). All mesoderm is derived from the primary mesen- intermediate mesoderm endoderm chyme that migrated through the notochord coelom (becomes urogenital ridge) primitive streak. Intermediate mesoderm (plus adjacent mesothelium lining the coelom) forms a urogenital ridge, which consists of a laterally-positioned nephrogenic cord (that forms kidneys & ureter) and a medially-positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Thus urinary & genital systems have a common embryonic origin; also, they share common ducts. NOTE: Urine production essentially requires an increased capillary surface area (glomeruli), epithelial tubules to collect plasma filtrate and extract desirable constituents, and a duct system to convey urine away from the body. Kidneys Bilateraly, three kid- mesonephric duct neys develop from the neph- metanephros pronephros rogenic cord. They develop mesonephric tubules chronologically in cranial- mesonephros caudal sequence, and are designated pro—, meso—, Nephrogenic Cord (left) and meta—, respectively. cloaca The pronephros and mesonephros develop similarly: the nephrogenic cord undergoes seg- mentation, segments become tubules, tubules drain into a duct & eventually tubules disintegrate. spinal ganglion 1] Pronephros—consists of (7-8) primitive tubules and a pronephric duct that grows caudally and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the neural tube mesonephric duct. (The pronephros is not functional, somite except in sheep.) notochord mesonephric NOTE tubule The mesonephros is the functional kidney for fish and am- aorta phibians. The metanephros is the functional kidney body of reptiles, birds, & mammals. -

The Derivatives of Three-Layered Embryo (Germ Layers)
HUMANHUMAN EMBRYOLOGYEMBRYOLOGY Department of Histology and Embryology Jilin University ChapterChapter 22 GeneralGeneral EmbryologyEmbryology FourthFourth week:week: TheThe derivativesderivatives ofof trilaminartrilaminar germgerm discdisc Dorsal side of the germ disc. At the beginning of the third week of development, the ectodermal germ layer has the shape of a disc that is broader in the cephalic than the caudal region. Cross section shows formation of trilaminar germ disc Primitive pit Drawing of a sagittal section through a 17-day embryo. The most cranial portion of the definitive notochord has formed. ectoderm Schematic view showing the definitive notochord. horizon =ectoderm hillside fields =neural plate mountain peaks =neural folds Cave sinks into mountain =neural tube valley =neural groove 7.1 Derivatives of the Ectodermal Germ Layer 1) Formation of neural tube Notochord induces the overlying ectoderm to thicken and form the neural plate. Cross section Animation of formation of neural plate When notochord is forming, primitive streak is shorten. At meanwhile, neural plate is induced to form cephalic to caudal end, following formation of notochord. By the end of 3rd week, neural folds and neural groove are formed. Neural folds fuse in the midline, beginning in cervical region and Cross section proceeding cranially and caudally. Neural tube is formed & invade into the embryo body. A. Dorsal view of a human embryo at approximately day 22. B. Dorsal view of a human embryo at approximately day 23. The nervous system is in connection with the amniotic cavity through the cranial and caudal neuropores. Cranial/anterior neuropore Neural fold heart Neural groove endoderm caudal/posterior neuropore A. -

Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E
1 Embryology of the Kidney Rizaldy Paz Scott | Yoshiro Maezawa | Jordan Kreidberg | Susan E. Quaggin CHAPTER OUTLINE MAMMALIAN KIDNEY DEVELOPMENT, 2 MOLECULAR GENETICS OF MODEL SYSTEMS TO STUDY KIDNEY NEPHROGENESIS, 22 DEVELOPMENT, 8 GENETIC ANALYSIS OF MAMMALIAN KIDNEY DEVELOPMENT, 15 KEY POINTS • The development of the kidney relies on reciprocal signaling and inductive interactions between neighboring cells. • Epithelial cells that comprise the tubular structures of the kidney are derived from two distinct cell lineages: the ureteric epithelia lineage that branches and gives rise to collecting ducts and the nephrogenic mesenchyme lineage that undergoes mesenchyme to epithelial transition to form connecting tubules, distal tubules, the loop of Henle, proximal tubules, parietal epithelial cells, and podocytes. • Nephrogenesis and nephron endowment requires an epigenetically regulated balance between nephron progenitor self-renewal and epithelial differentiation. • The timing of incorporation of nephron progenitor cells into nascent nephrons predicts their positional identity within the highly patterned mature nephron. • Stromal cells and their derivatives coregulate ureteric branching morphogenesis, nephrogenesis, and vascular development. • Endothelial cells track the development of the ureteric epithelia and establish the renal vasculature through a combination of vasculogenic and angiogenic processes. • Collecting duct epithelia have an inherent plasticity enabling them to switch between principal and intercalated cell identities. MAMMALIAN KIDNEY DEVELOPMENT The filtration function of the kidneys is accomplished by basic units called nephrons (Fig. 1.1). Humans on average have 1 million nephrons per adult kidney but the range of ANATOMIC OVERVIEW OF THE 4 MAMMALIAN KIDNEY total nephrons is highly variable across human populations. Each mouse kidney may contain up to 12,000–16,000 nephrons The kidney is a sophisticated, highly vascularized organ that depending on the strain.5 This wide range in nephron number plays a central role in overall body homeostasis. -
Genital System • Anatomically, the Genital System Is Subdivided Into
Genital system • Anatomically, the genital system is subdivided into: 1- sex glands ( Testis and Ovary). 2- duct system ( male or female duct system) 3- external genitalia ( scrotum and penis in male, vulva in female) . • Embryollogically, from the time of fertilization the sex of the embryo can be determined genetically (male XY and female XX). • In early stage of development the sex of embryo cannot be differentiated anatomically or histologically, this stage is termed indifferent stage. • Later on ,sex hormones induce differentiation into male or female and in the same time the structure of the other sex degenerate. Indifferent Stage:- • In this stage the sex of the embryo cannot be differentiated anatomically or histologically. • This stage includes development of : - - gonads. - duct system - external genitalia. A- Gonads 1. Due to enlargement of mesonephros, it bulges ventrolaterally into the coelum forming a longitudinal urogenital ridge. 2. This ridge subdivided longitudinally into a lateral urinary ridge and medial genital ridge. 2. The genital (gonadal) ridge or gonad results from proliferation of celomatic epithelium. 3. This celomatic epithelium forms sex cord which project in the underlying mesenchyme . 4. The primordial germ cells which are located between the endodermal cells of the yolk sac, migrate through the mesentery of the hind gut to be located in the sex cord of the gonads. 5. Therefore the gonad has three types of cells: -Primordial germ cells. -celomatic cells -mesenchymal cells. B- Duct system • Both male and female embryos have initially two pairs of genital ducts: Mesonephric and Paramesonephric. 1. Mesonephric (Wolffian) duct: • The Mesonephric duct and tubules remain after degeneration of mesonephros forming the male duct system. -

The Development of the Urogenital System in the Marsupialia, with Special Reference to Trichosurus Vulpecula Part I
THE DEVELOPMENT OF THE UROGENITAL SYSTEM IN THE MARSUPIALIA, WITH SPECIAL REFERENCE TO TRICHOSURUS VULPECULA PART I By GWYNNETH BUCHANAN, D.Sc. (MELB.) AND ELIZABETH A. FRASER, D.Sc. (LOND.) From the Embryological Laboratory, Department of Zoology, University of London, University College EDITED WITH AN INTRODUCTORY NOTE BY PROF. J. P. HILL, D.Sc., F.R.S. CONTENTS PAGE Introductory Note .35 Pronephros and Mesonephros 36 Discussion. 42 Metanephros and Ureter . .46 Discussion ..... 50 Cloaca . ... 52 Discussion .....68 Bladder . 70 Wolffian Duct. 71 Mullerian Duet . 74 Summary and Discussion . .82 Suprarenal Body . 87 Conclusions. 88 List of References . .91 Description of Plates . .93 INTRODUCTORY NOTE THE present memoir is the result of the independent investigations of the two authors whose names appear in the title. The research was originally undertaken at my suggestion by Miss Buchanan during her tenure of a travelling scholarship of the University of Melbourne in 1914, and was in- tended to afford as complete an account of the morphogenesis of the marsupial urogenital system as the available material would permit. Miss Buchanan devoted her attention more particularly to the earlier stages of development, concerning which practically nothing was known, and embodied her results in a thesis which, along with other contributions, was accepted for the degree of D.Sc. by the University of Melbourrne. This thesis has not been published and is incorporated in the present memoir. Owing to the ;3-2 36 G. Buchanan and E. A. Fraser limited time at her disposal, Miss Buchanan was unable to deal inl any detail with the later stages. -

Renal Development
RENAL DEVELOPMENT Jon Barasch M.D., Ph.D. Telephone: 305-1890 e-mail: [email protected] SUGGESTED READING: Larsen, 3rd edition, pp 265 (first three paragraphs) - 266, 268-276 and figure 10-10 LEARNING OBJECTIVES: You should be able to: 1. Describe the three kidneys that are produced during development and know what happens to each one. 2. Explain what is meant by ‘reciprocal induction’ and why it poses problems in interpreting experiments in developing kidney. 3. Describe the stages of nephron formation from the renal vesicle. 4. Discuss the regulators of mesenchymal to epithelial transition in the intermediate mesoderm and metanephric mesenchyme and name three molecules mediating conversion. 5. Describe branching morphogenesis and name the three patterns in the developing metanephros. 6. Discuss three key important ligands and their receptors. 7. Discuss the classification of congenital renal abnormalities that are associated with urological abnormalities and the possible underlying mechanisms for their association. SUMMARY: The urogenital system derives from mesenchymal cells by a process of conversion to epithelia. The development of the kidney relies on three mechanisms of epithelial morphogenesis. 1. Some newborn epithelia migrate extensively (Wolffian duct), 2. some undergo branching morphogenesis (ureteric bud) and 3. some produce highly segmented tubules (nephrons). GLOSSARY: Angiotensin II: your favorite vasoconstrictor and regulator of proximal tubule reclamation of NaCl and water by receptor type 1. Receptor type-2 modulates cell growth and seems to play a role in congenital abnormalities. Arcade: a tubule of ureteric bud that induces a few nephrons simultaneously. The nephrons join to a common drainage called a connecting tubule that feeds into the ureteric’s collecting duct. -
Embryology of Urogenital System
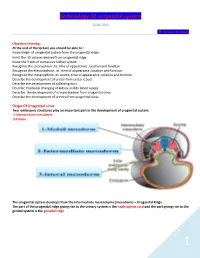
Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Objective learning : At the end of the lecture you should be able to : Know Origin of urogenital system from the urogenital ridge Enlist the Structures derived from urogenital ridge Know the 3 sets of successive kidney system Recognize the pronephron ,its time of appearance ,location and function Recognize the Mesonephron, its time of appearance ,location and function Recognize the metanephron ,its source, time of appearance ,location and function Describe the development of ureter from ureteric bud Describe the development of collecting duct Describe Positional changing of kidney and its blood supply Describe the development of urinary bladder from urogenital sinus Describe the development of urethra from urogenital sinus Origin Of Urogenital sinus Two embryonic structures play an important part in the development of urogenital system: 1-Intermediate mesoderm 2-Cloaca The urogenital system develops from the intermediate mesenchyme (mesoderm) – Urogenital Ridge. The part of the urogenital ridge giving rise to the urinary system is the nephrogenic cord and the part giving rise to the genital system is the gonadal ridge. 1 Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Development of urinary system: 3 sets of successive kidneys : Pronephron: These bilateral structures appear early in the fourth week. Segmented division of intermediate mesoderm form a few cell clusters and 5-7 pairs pronephric tubules in the cervical region. One end of the tubules opened at coelomic cavity and the other end opened in to pronephric duct 2 Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Function : The tubules transmits the waste product from coelomic cavity to the pronephric duct that runs caudally and open into the Cloaca .