From Hatching Into Fetal Life in the P E in the P E in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 19 Placentation and Maternal Recognition of Pregnancy
Blastulation Gap Junctions Lecture 19 Inner Cell Mass Zona Pellucida Placentation and Maternal Recognition of Pregnancy Trophectoderm Na+ [Na+] Animal Science 434 John J. Parrish H2O Tight Junctions Hatching Conceptus Growth Cow • Day 15, 1-2 mm Occurs in cow, pig and sheep Bovine • Day 18-19, 10-20 cm »9 - 11 days Spherical Embryonic Equine, Ovine Area »7 - 8 days Porcine Tubular Elongating »6 days Trophoblast Filamentous Mare remains spherical! Development of Porcine Conceptuses Elongated Day 15 Porcine from Day 10 to 12 Conceptus 5 mm Spherical 10 mm Spherical Inner Cell Mass 15 mm Tubular 150 mm Filamentous Embryo 1 Uterine Location of Elongating Pig Intrauterine Migration Ruminant Blastocyst Day 5 Corpus Luteum Bovine and Ovine Pig Intrauterine Migration Pig Intrauterine Migration Day 7 Day 12 Embryos become fixed Transuterine migration is rare in cow and ewe! Trans-uterine Migration in the Mare Gastrulation Begins Day 10 Inner Cell Mass Trophectoderm Formation Endoderm of Germ Fixation can Fixation occur in either on day Layers horn! 15 - 16 Blastocoele Corpus Cavity Luteum 2 Gastrulation Gastrulation Endoderrm Endoderrm Yolk Sack Yolk Sack Gastrula Gastrula Ectoderm Ectoderm Mesoderm Ectoderm Trophectoderm Trophectoderm Extraembryonic Coelom Yolk Sack Endoderm Endoderm Trophectoderm (Chorion) Germ Layers Placenta Formation Embryonic Amniotic Folds Ectoderm Mesoderm Endoderm Ectoderm » CNS » Circulatory » Digestive » Sense organs » Skeletal » Liver » Mammary » Muscle » Lungs glands » Reproductive » Pancreas Extraembryonic » -

Urinary System Intermediate Mesoderm
Urinary System Intermediate mesoderm lateral mesoderm: somite ectoderm neural NOTE: Intermediate mesoderm splanchnic groove somatic is situated between somites and lateral mesoderm (somatic and splanchnic mesoderm bordering the coelom). All mesoderm is derived from the primary mesen- intermediate mesoderm endoderm chyme that migrated through the notochord coelom (becomes urogenital ridge) primitive streak. Intermediate mesoderm (plus adjacent mesothelium lining the coelom) forms a urogenital ridge, which consists of a laterally-positioned nephrogenic cord (that forms kidneys & ureter) and a medially-positioned gonadal ridge (for ovary/testis & female/male genital tract formation). Thus urinary & genital systems have a common embryonic origin; also, they share common ducts. NOTE: Urine production essentially requires an increased capillary surface area (glomeruli), epithelial tubules to collect plasma filtrate and extract desirable constituents, and a duct system to convey urine away from the body. Kidneys Bilateraly, three kid- mesonephric duct neys develop from the neph- metanephros pronephros rogenic cord. They develop mesonephric tubules chronologically in cranial- mesonephros caudal sequence, and are designated pro—, meso—, Nephrogenic Cord (left) and meta—, respectively. cloaca The pronephros and mesonephros develop similarly: the nephrogenic cord undergoes seg- mentation, segments become tubules, tubules drain into a duct & eventually tubules disintegrate. spinal ganglion 1] Pronephros—consists of (7-8) primitive tubules and a pronephric duct that grows caudally and terminates in the cloaca. The tubules soon degenerate, but the pronephric duct persists as the neural tube mesonephric duct. (The pronephros is not functional, somite except in sheep.) notochord mesonephric NOTE tubule The mesonephros is the functional kidney for fish and am- aorta phibians. The metanephros is the functional kidney body of reptiles, birds, & mammals. -

The Derivatives of Three-Layered Embryo (Germ Layers)
HUMANHUMAN EMBRYOLOGYEMBRYOLOGY Department of Histology and Embryology Jilin University ChapterChapter 22 GeneralGeneral EmbryologyEmbryology FourthFourth week:week: TheThe derivativesderivatives ofof trilaminartrilaminar germgerm discdisc Dorsal side of the germ disc. At the beginning of the third week of development, the ectodermal germ layer has the shape of a disc that is broader in the cephalic than the caudal region. Cross section shows formation of trilaminar germ disc Primitive pit Drawing of a sagittal section through a 17-day embryo. The most cranial portion of the definitive notochord has formed. ectoderm Schematic view showing the definitive notochord. horizon =ectoderm hillside fields =neural plate mountain peaks =neural folds Cave sinks into mountain =neural tube valley =neural groove 7.1 Derivatives of the Ectodermal Germ Layer 1) Formation of neural tube Notochord induces the overlying ectoderm to thicken and form the neural plate. Cross section Animation of formation of neural plate When notochord is forming, primitive streak is shorten. At meanwhile, neural plate is induced to form cephalic to caudal end, following formation of notochord. By the end of 3rd week, neural folds and neural groove are formed. Neural folds fuse in the midline, beginning in cervical region and Cross section proceeding cranially and caudally. Neural tube is formed & invade into the embryo body. A. Dorsal view of a human embryo at approximately day 22. B. Dorsal view of a human embryo at approximately day 23. The nervous system is in connection with the amniotic cavity through the cranial and caudal neuropores. Cranial/anterior neuropore Neural fold heart Neural groove endoderm caudal/posterior neuropore A. -

Paraxial Mesoderm)
By DR. SANAA ALSHAARAWY DR. ESSAM ELDIN SALAMA OBJECTIVES : At the end of the lecture, the student should be able to describe : Changes in the bilaminar germ disc (embryonic plate). Formation of the secondary embryonic mesoderm (intraembryonic mesoderm). Formation of trilaminar germ disc. Formation of the primitive streake & notochord. Differantiation of intra-embryonic mesoderm. Implantation of the blastocyst is completed by the end of the 2nd week . As this process occurs, changes occur in the embryoblast that produce a bilaminar embryonic disc. The embryonic disc gives rise to the germ layers that form all tissues & organs of the embryo. Extraembryonic structures forming during the 2nd week are : the amniotic cavity, amnion, yolk sac, and connecting stalk. By the (8th) day: The Inner Cell Mass (Embryoblast)is differentiated into a bilaminar plate of cells composed of Two layers : (A) Epiblast High columnar cells adjacent to the amniotic cavity. (B) Hypoblast Small cuboidal cells adjacent to the blastocyst cavity (Yolk Sac). A loose connective tissue, arises from the yolk sac. It fills all the space between the trophoblast externally and the exocoelomic membrane & amnion internally. It surrounds the amnion and yolk sac. Multiple spaces appear within the Extraembryonic mesoderm. These spaces fuse and form the Extraembryonic Coelom. It surrounds the amnion and yolk sac. It is the process through which the Bilaminar embryonic disc is changed into a Trilaminar disc, as a new tissue (2ry or intraembryonic mesoderm) appears between the ectoderm and endoderm. Now the embryonic disc is formed of 3 layers: Embryonic Ectoderm Intraembryonic Mesoderm. Embryonic Endoderm. Cells in these layers will give rise to all tissues and organs of the embryo. -

Development of Chick Development of Chick
Unit 15 Development of Chick UNIT 15 DEVELOPMENT OF CHICK StructureStructureStructure 15.1 Introduction Fully Formed Gastrula Objectives 15.6 Neurulation in Chick 15.2 Structure of Egg of Chick Mechanisms of Neural Plate 15.3 Fertilisation Formation 15.4 Cleavage and Blastulation Morphogenesis of Mesodermal Derivatives 15.5 Gastrulation 15.7 Folding of Embryo Role of Hypoblast 15.8 Development of Extra- Fate Map Embryonic Membranes The Gastrulation Process: Development of Amnion and Formation of Primitive Streak Chorion Completion of Endoderm Development of Allantois Regression of Primitive Streak 15.9 Hatching Epiboly of Ectoderm 15.10 Summary Characteristic Features of 15.11 Terminal Questions Avian Gastrulation 15.12 Answers Comparison with Amphibian Gastrulation 15.1 INTRODUCTION Different animals have evolved a variety of strategies of development. However, since all animals are related, the basic mechanism of early development has been conserved in the course of evolution, and so there are some important similarities in early embryonic development of all metazoan animals as you have already learnt in Block 3. This unit speaks about development of chick as an example of an amniote organism. Recall that amniotes are those vertebrates (reptiles, birds and mammals) that have a water sac or amnion surrounding the developing 163 Block 4 Developmental Biology of Vertebrates-II organism protecting it from the external environment. Chick has been one of the first model organisms to be studied in detail as it is easy to maintain and large enough to be manipulated surgically and genetically during all stages of development. You will study about strictly coordinated sequential changes that take place during the course of chick development viz. -

Extracellular Vesicles Secreted During Blastulation Show Viability of Bovine Embryos
158 6 REPRODUCTIONRESEARCH Extracellular vesicles secreted during blastulation show viability of bovine embryos Edwin A Mellisho, Mario A Briones, Alejandra E Velásquez, Joel Cabezas, Fidel O Castro and Lleretny Rodríguez-Álvarez Laboratory of Animal Biotechnology, Department of Animal Science, Faculty of Veterinary Science, Universidad de Concepcion, Chillan, Chile Correspondence should be addressed to L Rodríguez-Álvarez; Email: [email protected] Abstract Extracellular vesicles (EVs) secreted by blastocysts may be clinically relevant, as indicator of embryo viability on in vitro fertilization. We tested if the characteristics of EVs secreted during blastulation are related to embryo viability. Morulae were individually cultured in SOF media depleted of EVs until day 7.5 post IVF. Viable embryos were determined by a system of extended in vitro culture of bovine embryos until day 11 (post-hatching development). Afterward, a retrospective classification of blastocyst and culture media was performed based on blastulation time (early blastulation (EB) or late blastulation (LB)) and post-hatching development at day 11 (viable (V) or non-viable embryo (NV)). A total of 254 blastocysts and their culture media were classified in four groups (V-EB, NV-EB, V-LB, NV-LB). Group V-EB had a larger blastocyst diameter (170.8 μm), higher proportion of good-quality blastocysts (77%) and larger mean size of population of EVs (122.9 nm), although the highest concentration of EVs (5.75 × 109 particles/mL) were in group NV-EB. Furthermore, small RNA sequencing detected two biotypes, miRNA (86–91%) and snoRNA (9–14%), with a total of 182 and 32 respectively. In differential expression analysis of miRNAs between V versus NV blastocysts, there were 12 miRNAs upregulated and 15 miRNAs downregulated. -

Cleavage: Types and Patterns Fertilization …………..Cleavage
Cleavage: Types and Patterns Fertilization …………..Cleavage • The transition from fertilization to cleavage is caused by the activation of mitosis promoting factor (MPF). Cleavage • Cleavage, a series of mitotic divisions whereby the enormous volume of egg cytoplasm is divided into numerous smaller, nucleated cells. • These cleavage-stage cells are called blastomeres. • In most species the rate of cell division and the placement of the blastomeres with respect to one another is completely under the control of the proteins and mRNAs stored in the oocyte by the mother. • During cleavage, however, cytoplasmic volume does not increase. Rather, the enormous volume of zygote cytoplasm is divided into increasingly smaller cells. • One consequence of this rapid cell division is that the ratio of cytoplasmic to nuclear volume gets increasingly smaller as cleavage progresses. • This decrease in the cytoplasmic to nuclear volume ratio is crucial in timing the activation of certain genes. • For example, in the frog Xenopus laevis, transcription of new messages is not activated until after 12 divisions. At that time, the rate of cleavage decreases, the blastomeres become motile, and nuclear genes begin to be transcribed. This stage is called the mid- blastula transition. • Thus, cleavage begins soon after fertilization and ends shortly after the stage when the embryo achieves a new balance between nucleus and cytoplasm. Cleavage Embryonic development Cleavage 2 • Division of first cell to many within ball of same volume (morula) is followed by hollowing -
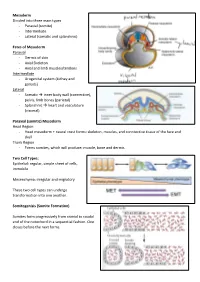
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -
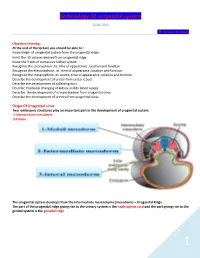
Embryology of Urogenital System
Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Objective learning : At the end of the lecture you should be able to : Know Origin of urogenital system from the urogenital ridge Enlist the Structures derived from urogenital ridge Know the 3 sets of successive kidney system Recognize the pronephron ,its time of appearance ,location and function Recognize the Mesonephron, its time of appearance ,location and function Recognize the metanephron ,its source, time of appearance ,location and function Describe the development of ureter from ureteric bud Describe the development of collecting duct Describe Positional changing of kidney and its blood supply Describe the development of urinary bladder from urogenital sinus Describe the development of urethra from urogenital sinus Origin Of Urogenital sinus Two embryonic structures play an important part in the development of urogenital system: 1-Intermediate mesoderm 2-Cloaca The urogenital system develops from the intermediate mesenchyme (mesoderm) – Urogenital Ridge. The part of the urogenital ridge giving rise to the urinary system is the nephrogenic cord and the part giving rise to the genital system is the gonadal ridge. 1 Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Development of urinary system: 3 sets of successive kidneys : Pronephron: These bilateral structures appear early in the fourth week. Segmented division of intermediate mesoderm form a few cell clusters and 5-7 pairs pronephric tubules in the cervical region. One end of the tubules opened at coelomic cavity and the other end opened in to pronephric duct 2 Embryology Of urogenital system 2018-2019 DR. Hassna B. Jawad Function : The tubules transmits the waste product from coelomic cavity to the pronephric duct that runs caudally and open into the Cloaca . -

Formation of Germ Layers (Second & Third Week of Development)
8.12.2014 Formation of Germ Layers (Second & Third week of Development) Dr. Archana Rani Associate Professor Department of Anatomy KGMU UP, Lucknow Day 8 • Blastocyst is partially embedded in the endometrial stroma. • Trophoblast differentiates into 2 layers: (i) Cytotrophoblast (ii) Syncytiotrophoblast • Cytotrophoblast shows mitotic division. Day 8 • Cells of inner cell mass (embryoblast) also differentiate into 2 layers: (i) Hypoblast layer (ii) Epiblast layer • Formation of amniotic cavity and embryonic disc. Day 9 • The blastocyst is more deeply embedded in the endometrium. • The penetration defect in the surface epithelium is closed by a fibrin coagulum. Day 9 • Large no. of vacuoles appear in syncytiotrophoblast which fuse to form lacunae which contains embryotroph. Day 9 • Hypoblast forms the roof of the exocoelomic cavity (primary yolk sac). • Heuser’s (exocoelomic membrane) • Extraembryonic mesoderm Day 11 & 12 • Formation of lacunar networks • Extraembryonic coelom (chorionic cavity) • Extraembryonic somatic mesoderm • Extraembryonic splanchnic mesoderm • Chorion Day 13 • Implantation bleeding • Villous structure of trophoblast. • Formation of Primary villi • Secondary (definitive) yolk sac • Chorionic plate (extraembronic mesoderm with cytotrophoblast) Third week of Development • Gastrulation (formation of all 3 germ layers) • Formation of primitive streak • Formation of notochord • Differentiation of 3 germ layers from Bilaminar to Trilaminar germ disc Formation of Primitive Streak (PS) • First sign of gastrulation • On 15th day • Primitive node • Primitive pit • Formation of mesenchyme on 16th day • Formation of embryonic endoderm • Intraembryonic mesoderm • Ectoderm • Epiblast is the source of all 3 germ layers Fate of Primitive Streak • Continues to form mesodermal cells upto early part of 4th week • Normally, the PS degenerates & diminishes in size. -

Developmental Biology
DEVELOPMENTAL BIOLOGY DR. THANUJA A MATHEW DEVELOPMENT OF AMPHIOXUS • Eggs- 0.02mm in diameter, microlecithal, isolecithal Cleavage- Holoblastic equal • 1st & 2nd Cleavage- Meridional • 3rd Cleavage-lattitudinal- 4 micromeres& 4 macromeres are produced • 4th Cleavage-Meridional & double • 5th Cleavage- lattitudinal & double • 6th Cleavage onwards irregular 2 BLASTULATION • Blastocoel is filled with jelly like subtance which exerts pressure on blastomeres to become blastoderm • Blastula – equal coeloblastula but contains micromeres in animal hemisphere & macromeres in vegetal hemisphere 3 4 GASTRULATION • The process of formation of double layered gastrula. • The outer layer- ectoderm& inner layer- median notochord flanked by mesoderm. The remaining cells are endodermal cells. • Begin after 6 hrs with flattening of prospective endodermal area. • Characterized by morphogenetic movements & antero posterior elongation 6 7 Morphogenetic movements • 1. Invagination of P.endodermal cells into blastocoel reducing the cavity and a new cavity is produced- gastrocoel or archenteron which opens to outside through blastopore • Blastopore has a circular rim - lip • P.notochord lie in the dorsal lip • P.mesoderm lie in the ventro lateral lip 8 • 2. Involution – rolling in of notochord • 3.Covergence- mesoderm converge towards ventro lateral lip and involute in • 4.Epiboly- Proliferation of ectodermal cell over the entire gastrula 9 Antero posterior elongation • Notochord – long median band • Mesoderm- two horns on either sides of notochord Ectoderm • Neurectoderm -elongate into a median band above notochord • Epidermal ectoderm - cover the rest of the gastrula 10 NEURULATION • 1.Neurectoderm thickens to form neural plate. • 2. Neural plate sinks down • 3. Edges of neural plate rise up as neural folds • 4.Neural folds meet and fuse in the mid line • 5. -

Imaging of Urachal Anomalies
Abdominal Radiology (2019) 44:3978–3989 https://doi.org/10.1007/s00261-019-02205-x SPECIAL SECTION : UROTHELIAL DISEASE Imaging of urachal anomalies Suryakala Buddha1 · Christine O. Menias2 · Venkata S. Katabathina1 Published online: 3 September 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Urachal anomalies are classifed into four types depending on the level of persistence of the embryonic urachal remnants between the urinary bladder and the umbilicus: patent urachus, umbilical–urachal sinus, urachal cyst, and vesico-urachal diverticulum. Due to the increasing use of cross-sectional imaging, urachal anomalies are frequently detected as incidental fndings. Imaging plays a pivotal role in the initial diagnosis, evaluation of complications, treatment follow-up, and long-term surveillance of patients with urachal anomalies. Diferent urachal anomalies demonstrate characteristic imaging features that aid in a timely diagnosis and guide treatment. A patent urachus is visualized as an elongated tubular structure between the umbilicus and the urinary bladder. While umbilical–urachal sinus appears as focal dilatation at the umbilical end of the urachal remnant, the vesico-urachal diverticulum presents as a focal outpouching of the urinary bladder at anterosuperior aspect. Urachal cysts are identifed as midline fuid-flled sacs most frequently located near the dome of the urinary bladder. Untreated urachal anomalies could progress into potential complications, including infection and malignancy. Knowledge regarding