Emergency and Combat First Aid» Module № 1 Emergency and Combat First Aid Topic 7 Means of Mass Destruction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RADIATION EFFECTS and SOURCES What Is Radiation? What Does Radiation Do to Us? Where Does Radiation Come From?
RADIATION EFFECTS and SOURCES What is radiation? What does radiation do to us? Where does radiation come from? United Nations Environment Programme RADIATION EFFECTS and SOURCES What is radiation? What does radiation do to us? Where does radiation come from? United Nations Environment Programme DISCLAIMER This publication is largely based on the findings of the United Nations Scientific Committee on the Effects of Atomic Radiation, a subsidiary body of the United Nations General Assembly and for which the United Nations Environment Pro- gramme provides the secretariat. This publication does not necessarily r epresent the views of the Scientific Committee or of the United Nations Environment Programme. The designations employed and the presentation of the material in this publica- tion do not imply the expression of any opinion whatsoever on the part of the United Nations Environment Programme concerning the legal status of any country, territory, city or area or of its authorities, or concerning delimitation of its frontiers or boundaries. This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. The United Nations Environment Programme would appreciate receiving a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from the United Nations Environment Programme. The United Nations Environment Programme promotes environmentally sound practices globally and in its own activities. This publication was printed on recycled paper, 100 per cent chlorine free. -

Absorbed Dose in Radioactive Media Outline Introduction Radiation Equilibrium Charged-Particle Equilibrium Limiting Cases
Outline • General dose calculation considerations, Absorbed Dose in Radioactive absorbed fraction Media • Radioactive disintegration processes and associated dose deposition – Alpha disintegration Chapter 5 – Beta disintegration – Electron-capture transitions F.A. Attix, Introduction to Radiological – Internal conversion Physics and Radiation Dosimetry • Summary Introduction Radiation equilibrium • We are interested in calculating the absorbed dose in a. The atomic composition radioactive media, applicable to cases of of the medium is – Dose within a radioactive organ homogeneous – Dose in one organ due to radioactive source in another b. The density of the organ medium is • If conditions of CPE or RE are satisfied, dose homogeneous c. The radioactive source calculation is straightforward is uniformly distributed • Intermediate situation is more difficult but can be d. No external electric or handled at least in approximations magnetic fields are present Charged-particle equilibrium Limiting cases • Emitted radiation typically includes both • Each charged particle of a given type and photons (longer range) and charged energy leaving the volume is replaced by an particles (shorter range) identical particle of the same energy entering • Assume the conditions for RE are satisfied the volume • Consider two • Existence of RE is sufficient condition for CPE limited cases based • Even if RE does not exist CPE may still exist on the size of the (for a very large or a very small volume) radioactive object 1 Limiting cases: small object Limiting -

Development of Chemical Dosimeters Development Of
SUDANSUDAN ACADEMYAGADEMY OFOF SCIENCES(SAS)SGIENGES(SAS) ATOMICATOMIC ENERGYEhTERGYRESEARCHESRESEARCHES COORDINATIONCOORDII\rATI ON COUNCILCOUNCIL - Development of Chemical Dosimeters A dissertation Submitted in a partial Fulfillment of the Requirement forfbr Diploma Degree in Nuclear Science (Chemistry) By FareedFadl Alla MersaniMergani SupervisorDr K.S.Adam MurchMarch 2006 J - - - CONTENTS Subject Page -I - DedicationDedication........ ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... ... I Acknowledgement ... '" ... ... ... ... ... ... '" ... ... ... ... '" ... '" ....... .. 11II Abstract ... ... ... '" ... ... ... '" ... ... ... ... -..... ... ... ... ... ... ..... III -I Ch-lch-1 DosimetryDosimefry - 1-1t-l IntroductionLntroduction . 1I - 1-2t-2 Principle of Dosimetry '" '" . 2 1-3l-3 DosimetryDosimefiySystems . 3J 1-3-1l-3-l primary standard dosimeters '" . 4 - 1-3-2l-3-Z Reference standard dosimeters ... .. " . 4 1-3-3L-3-3 Transfer standard dosimeters ... ... '" . 4 1-3-4t-3-4 Routine dosimeters . 5 1-4I-4 Measurement of absorbed dose . 6 1-5l-5 Calibration of DosimetryDosimetrvsystemsvstem '" . 6 1-6l-6 Transit dose effects . 8 Ch-2ch-2 Requirements of chemical dosimeters 2-12-l Introduction ... ... ... .............................................. 111l 2-2 Developing of chemical dosimeters ... ... .. ....... ... .. ..... 12t2 2-3 Classification of Dosimetry methods.methods .......................... 14l4 2-4 RequirementsRequiremsnts of ideal chemical dosimeters ,. ... 15 2-5 Types of chemical system . -

The International Commission on Radiological Protection: Historical Overview
Topical report The International Commission on Radiological Protection: Historical overview The ICRP is revising its basic recommendations by Dr H. Smith Within a few weeks of Roentgen's discovery of gamma rays; 1.5 roentgen per working week for radia- X-rays, the potential of the technique for diagnosing tion, affecting only superficial tissues; and 0.03 roentgen fractures became apparent, but acute adverse effects per working week for neutrons. (such as hair loss, erythema, and dermatitis) made hospital personnel aware of the need to avoid over- Recommendations in the 1950s exposure. Similar undesirable acute effects were By then, it was accepted that the roentgen was reported shortly after the discovery of radium and its inappropriate as a measure of exposure. In 1953, the medical applications. Notwithstanding these observa- ICRU recommended that limits of exposure should be tions, protection of staff exposed to X-rays and gamma based on consideration of the energy absorbed in tissues rays from radium was poorly co-ordinated. and introduced the rad (radiation absorbed dose) as a The British X-ray and Radium Protection Committee unit of absorbed dose (that is, energy imparted by radia- and the American Roentgen Ray Society proposed tion to a unit mass of tissue). In 1954, the ICRP general radiation protection recommendations in the introduced the rem (roentgen equivalent man) as a unit early 1920s. In 1925, at the First International Congress of absorbed dose weighted for the way different types of of Radiology, the need for quantifying exposure was radiation distribute energy in tissue (called the dose recognized. As a result, in 1928 the roentgen was equivalent in 1966). -

Pediatric Considerations Before, During, and After Radiological Or Nuclear Emergencies
TECHNICAL REPORT Pediatric Considerations Before, During,Martha S. Linet, MD, MPH, and a, b Ziad Kazzi, After MD, c, d Jerome A. RadiologicalPaulson, MD, FAAP, e COUNCIL ON ENVIRONMENTAL HEALTH or Nuclear Emergencies Infants, children, and adolescents can be exposed unexpectedly to ionizing abstract radiation from nuclear power plant events, improvised nuclear or radiologic dispersal device explosions, or inappropriate disposal of radiotherapy equipment. Children are likely to experience higher external and internal radiation exposure levels than adults because of their smaller body and aRadiation Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland; bAgency for organ size and other physiologic characteristics as well as their tendency to Toxic Substances and Disease Registry, Centers for Disease Control pick up contaminated items and consume contaminated milk or foodstuffs. and Prevention, Atlanta, Georgia; cNational Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, Georgia; This technical report accompanies the revision of the 2003 American dDepartment of Emergency Medicine, Emory University, Atlanta, Georgia; and eDepartment of Pediatrics, School of Medicine and Health Academy of Pediatrics policy statement on pediatric radiation emergencies Sciences, and Department of Environmental and Occupational Health, by summarizing newer scientific data from studies of the Chernobyl and the Milken Institute School of Public Health, George Washington University, Washington, District of Columbia Fukushima Daiichi nuclear power plant events, use of improvised radiologic Drs Linet and Kazzi contributed much of the technical information in dispersal devices, exposures from inappropriate disposal of radiotherapy this report, and Dr Paulson was responsible for drafting the document; equipment, and potential health effects from residential proximity to and all authors approved the final manuscript as submitted. -

Metabolomics in Radiation Biodosimetry: Current Approaches and Advances
H OH metabolites OH Review Metabolomics in Radiation Biodosimetry: Current Approaches and Advances Merriline M. Satyamitra 1,*, David R. Cassatt 1, Brynn A. Hollingsworth 1, Paul W. Price 2, Carmen I. Rios 1, Lanyn P. Taliaferro 1, Thomas A. Winters 1 and Andrea L. DiCarlo 1 1 Radiation and Nuclear Countermeasures Program (RNCP), Division of Allergy, Immunology and Transplantation (DAIT), and National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), 5601 Fishers Lane, Rockville, MD 20852, USA; [email protected] (D.R.C.); [email protected] (B.A.H.); [email protected] (C.I.R.); [email protected] (L.P.T.); [email protected] (T.A.W.); [email protected] (A.L.D.) 2 Office of Regulatory Affairs, Division of Allergy, Immunology and Transplantation (DAIT), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), 5601 Fishers Lane, Rockville, MD 20852, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-240-669-5432 Received: 1 July 2020; Accepted: 6 August 2020; Published: 11 August 2020 Abstract: Triage and medical intervention strategies for unanticipated exposure during a radiation incident benefit from the early, rapid and accurate assessment of dose level. Radiation exposure results in complex and persistent molecular and cellular responses that ultimately alter the levels of many biological markers, including the metabolomic phenotype. Metabolomics is an emerging field that promises the determination of radiation exposure by the qualitative and quantitative measurements of small molecules in a biological sample. This review highlights the current role of metabolomics in assessing radiation injury, as well as considerations for the diverse range of bioanalytical and sampling technologies that are being used to detect these changes. -

Radiation Glossary
Radiation Glossary Activity The rate of disintegration (transformation) or decay of radioactive material. The units of activity are Curie (Ci) and the Becquerel (Bq). Agreement State Any state with which the U.S. Nuclear Regulatory Commission has entered into an effective agreement under subsection 274b. of the Atomic Energy Act of 1954, as amended. Under the agreement, the state regulates the use of by-product, source, and small quantities of special nuclear material within said state. Airborne Radioactive Material Radioactive material dispersed in the air in the form of dusts, fumes, particulates, mists, vapors, or gases. ALARA Acronym for "As Low As Reasonably Achievable". Making every reasonable effort to maintain exposures to ionizing radiation as far below the dose limits as practical, consistent with the purpose for which the licensed activity is undertaken. It takes into account the state of technology, the economics of improvements in relation to state of technology, the economics of improvements in relation to benefits to the public health and safety, societal and socioeconomic considerations, and in relation to utilization of radioactive materials and licensed materials in the public interest. Alpha Particle A positively charged particle ejected spontaneously from the nuclei of some radioactive elements. It is identical to a helium nucleus, with a mass number of 4 and a charge of +2. Annual Limit on Intake (ALI) Annual intake of a given radionuclide by "Reference Man" which would result in either a committed effective dose equivalent of 5 rems or a committed dose equivalent of 50 rems to an organ or tissue. Attenuation The process by which radiation is reduced in intensity when passing through some material. -

ACUTE RADIATION SYNDROME: Diagnosis and Treatment
ACUTE RADIATION SYNDROME: Diagnosis and Treatment Badria Al Hatali, MD Medical Toxicologist Department of Environmental and Occupational Health MOH - Oman Objectives Provide a review of radiation basics and acute radiation sickness Discuss diagnostic tools and triage tools for Acute Radiation Syndromes Discuss management of Acute Radiation Syndromes Energy traveling over a distance as Waves Particles • Gamma rays • Alpha • X-rays • Beta • Radio waves • neurons Non-ionizing vs Ionizing Radiation • High energy • Low energy • Removes orbital electrons • Does not remove orbital from atoms > DNA electrons from atom damage Radioactive Decay Process to Remove excess energy from atomic nuclei Nuclei emit rays or particles to decrease nuclear energy Radioactive materials have unstable nuclei with excess energy Ionizing Radiation Dose • Radiation absorb dose (RAD): the amount of energy absorbed by the body. 1 cGy = 0.01 J/kg (USA) • Gray (Gy): expressed as absorbed energy per unit mass of tissue. 100 rad =100 cGy =1 J/kg (SI) • Roentgen Equivalent Man (REM) relates the absorbed dose in human tissue to the effective biological damage of the radiation (USA) • Sievert (Sv): the absorbed dose in human tissue to the effective biological damage of the radiation (SI) Radioactivity Biological And Effective Half-lives Biological half-life is the time to remove half of radioactive element from body Effective half-life is the combined effect of radioactive decay & biological elimination Effective half-life is always shorter than either physical or biological half-lives Biological Effects of Ionizing Radiation Direct damage Chromosome Other biochemical E.g. alpha and beta particles Indirect damage Chemical changes due to radiolysis of water in cell E.g. -

11. Dosimetry Fundamentals
Outline • Introduction Dosimetry Fundamentals • Dosimeter model • Interpretation of dosimeter measurements Chapter 11 – Photons and neutrons – Charged particles • General characteristics of dosimeters F.A. Attix, Introduction to Radiological Physics and Radiation Dosimetry • Summary Introduction Dosimeter • Radiation dosimetry deals with the determination • A dosimeter can be generally defined as (i.e., by measurement or calculation) of the any device that is capable of providing a absorbed dose or dose rate resulting from the interaction of ionizing radiation with matter reading R that is a measure of the absorbed • Other radiologically relevant quantities are dose Dg deposited in its sensitive volume V exposure, kerma, fluence, dose equivalent, energy by ionizing radiation imparted, etc. can be determined • If the dose is not homogeneous • Measuring one quantity (usually the absorbed dose) another one can be derived through throughout the sensitive calculations based on the defined relationships volume, then R is a measure of mean value Dg Dosimeter Simple dosimeter model • Ordinarily one is not interested in measuring • A dosimeter can generally be considered as the absorbed dose in a dosimeter’s sensitive consisting of a sensitive volume V filled with a volume itself, but rather as a means of medium g, surrounded by a wall (or envelope, determining the dose (or a related quantity) for container, etc.) of another medium w having a another medium in which direct measurements thickness t 0 are not feasible • A simple dosimeter can be -
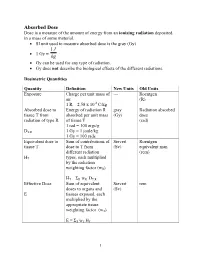
Absorbed Dose Dose Is a Measure of the Amount of Energy from an Ionizing Radiation Deposited in a Mass of Some Material
Absorbed Dose Dose is a measure of the amount of energy from an ionizing radiation deposited in a mass of some material. • SI unit used to measure absorbed dose is the gray (Gy). 1J • 1 Gy = kg • Gy can be used for any type of radiation. • Gy does not describe the biological effects of the different radiations. Dosimetric Quantities Quantity Definition New Units Old Units Exposure Charge per unit mass of --- Roentgen air (R) 1 R = 2.58 x 10-4 C/kg Absorbed dose to Energy of radiation R gray Radiation absorbed tissue T from absorbed per unit mass (Gy) dose radiation of type R of tissue T (rad) 1 rad = 100 ergs/g DT,R 1 Gy = 1 joule/kg 1 Gy = 100 rads Equivalent dose to Sum of contributions of Sievert Roentgen tissue T dose to T from (Sv) equivalent man different radiation (rem) HT types, each multiplied by the radiation weighting factor (wR) HT = ΣR wR DT,R Effective Dose Sum of equivalent Sievert rem doses to organs and (Sv) E tissues exposed, each multiplied by the appropriate tissue weighting factor (wT) E = ΣT wT HT 1 Radiological Protection For practical purposes of assessing and regulating the hazards of ionizing radiation to workers and the general population, weighting factors (previously called quality factors, Q) are used. A radiation weighting factor is an estimate of the effectiveness per unit dose of the given radiation relative a to low-LET standard. Weighting factors are dimensionless multiplicative factors used to convert physical dose (Gy) to equivalent dose (Sv) ; i.e., to place biological effects from exposure to different types of radiation on a common scale. -

Radiological Information
RADIOLOGICAL INFORMATION Frequently Asked Questions Radiation Information A. Radiation Basics 1. What is radiation? Radiation is a form of energy. It is all around us. It is a type of energy in the form of particles or electromagnetic rays that are given off by atoms. The type of radiation we are concerned with, during radiation incidents, is “ionizing radiation”. Radiation is colorless, odorless, tasteless, and invisible. 2. What is radioactivity? It is the process of emission of radiation from a material. 3. What is ionizing radiation? It is a type of radiation that has enough energy to break chemical bonds (knocking out electrons). 4. What is non-ionizing radiation? Non-ionizing radiation is a type of radiation that has a long wavelength. Long wavelength radiations do not have enough energy to "ionize" materials (knock out electrons). Some types of non-ionizing radiation sources include radio waves, microwaves produced by cellular phones, microwaves from microwave ovens and radiation given off by television sets. 5. What types of ionizing radiation are there? Three different kinds of ionizing radiation are emitted from radioactive materials: alpha (helium nuclei); beta (usually electrons); x-rays; and gamma (high energy, short wave length light). • Alpha particles stop in a few inches of air, or a thin sheet of cloth or even paper. Alpha emitting materials pose serious health dangers primarily if they are inhaled. • Beta particles are easily stopped by aluminum foil or human skin. Unless Beta particles are ingested or inhaled they usually pose little danger to people. • Gamma photons/rays and x-rays are very penetrating. -

Ionizing Radiation in Earth's Atmosphere and in Space Near Earth May 2011 6
Federal Aviation Administration DOT/FAA/AM-11/9 Office of Aerospace Medicine Washington, DC 20591 Ionizing Radiation in Earth’s Atmosphere and in Space Near Earth Wallace Friedberg Kyle Copeland Civil Aerospace Medical Institute Federal Aviation Administration Oklahoma City, OK 73125 May 2011 Final Report OK-11-0024-JAH NOTICE This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The United States Government assumes no liability for the contents thereof. ___________ This publication and all Office of Aerospace Medicine technical reports are available in full-text from the Civil Aerospace Medical Institute’s publications Web site: www.faa.gov/library/reports/medical/oamtechreports Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT/FAA/AM-11/9 4. Title and Subtitle 5. Report Date Ionizing Radiation in Earth's Atmosphere and in Space Near Earth May 2011 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Friedberg W, Copeland K 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) FAA Civil Aerospace Medical Institute P.O. Box 25082 11. Contract or Grant No. Oklahoma City, OK 73125 12. Sponsoring Agency name and Address 13. Type of Report and Period Covered Office of Aerospace Medicine Federal Aviation Administration 800 Independence Ave., S.W. Washington, DC 20591 14. Sponsoring Agency Code 15. Supplemental Notes 16. Abstract The Civil Aerospace Medical Institute of the FAA is charged with identifying health hazards in air travel and in commercial human space travel.