Office of Radiation Protection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Derived From: Human Research Program Integrated Research Plan, Revision A, (January 2009)
Glossary derived from: Human Research Program Integrated Research Plan, Revision A, (January 2009). National Aeronautics and Space Administration, Johnson Space Center, Houston, Texas 77058, pages 232-280. Report No. 153: Information Needed to Make Radiation Protection Recommendations for Space Missions Beyond Low-Earth Orbit (2006). National Council on Radiation Protection and Measurements, pages 309-318. Reprinted with permission of the National Council on Radiation Protection and Measurements, http://NCRPonline.org . Managing Space Radiation Risk in the New Era of Space Exploration (2008). Committee on the Evaluation of Radiation Shielding for Space Exploration, National Research Council. National Academies Press, pages 111-118. -A- AAPM: American Association of Physicists in Medicine. absolute risk: Expression of excess risk due to exposure as the arithmetic difference between the risk among those exposed and that obtaining in the absence of exposure. absorbed dose (D): Average amount of energy imparted by ionizing particles to a unit mass of irradiated material in a volume sufficiently small to disregard variations in the radiation field but sufficiently large to average over statistical fluctuations in energy deposition, and where energy imparted is the difference between energy entering the volume and energy leaving the volume. The same dose has different consequences depending on the type of radiation delivered. Unit: gray (Gy), equivalent to 1 J/kg. ACE: Advanced Composition Explorer Mission, launched in 1997 and orbiting the L1 libration point to sample energetic particles arriving from the Sun and interstellar and galactic sources. It also provides continuous coverage of solar wind parameters and solar energetic particle intensities (space weather). When reporting space weather, it can provide an advance warning (about one hour) of geomagnetic storms that can overload power grids, disrupt communications on Earth, and present a hazard to astronauts. -

Cherenkov Radiation
TheThe CherenkovCherenkov effecteffect A charged particle traveling in a dielectric medium with n>1 radiates Cherenkov radiation B Wave front if its velocity is larger than the C phase velocity of light v>c/n or > 1/n (threshold) A β Charged particle The emission is due to an asymmetric polarization of the medium in front and at the rear of the particle, giving rise to a varying electric dipole momentum. dN Some of the particle energy is convertedγ = 491into light. A coherent wave front is dx generated moving at velocity v at an angle Θc If the media is transparent the Cherenkov light can be detected. If the particle is ultra-relativistic β~1 Θc = const and has max value c t AB n 1 cosθc = = = In water Θc = 43˚, in ice 41AC˚ βct βn 37 TheThe CherenkovCherenkov effecteffect The intensity of the Cherenkov radiation (number of photons per unit length of particle path and per unit of wave length) 2 2 2 2 2 Number of photons/L and radiation d N 4π z e 1 2πz 2 = 2 1 − 2 2 = 2 α sin ΘC Wavelength depends on charge dxdλ hcλ n β λ and velocity of particle 2πe2 α = Since the intensity is proportional to hc 1/λ2 short wavelengths dominate dN Using light detectors (photomultipliers)γ = sensitive491 in 400-700 nm for an ideally 100% efficient detector in the visibledx € 2 dNγ λ2 d Nγ 2 2 λ2 dλ 2 2 11 1 22 2 d 2 z sin 2 z sin 490393 zz sinsinΘc photons / cm = ∫ λ = π α ΘC ∫ 2 = π α ΘC 2 −− 2 = α ΘC λ1 λ1 dx dxdλ λ λλ1 λ2 d 2 N d 2 N dλ λ2 d 2 N = = dxdE dxdλ dE 2πhc dxdλ Energy loss is about 104 less hc 2πhc than 2 MeV/cm in water from € -

Nuclear Glossary
NUCLEAR GLOSSARY A ABSORBED DOSE The amount of energy deposited in a unit weight of biological tissue. The units of absorbed dose are rad and gray. ALPHA DECAY Type of radioactive decay in which an alpha ( α) particle (two protons and two neutrons) is emitted from the nucleus of an atom. ALPHA (ααα) PARTICLE. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are a highly ionizing form of particle radiation, and have low penetration. Alpha particles are emitted by radioactive nuclei such as uranium or radium in a process known as alpha decay. Owing to their charge and large mass, alpha particles are easily absorbed by materials and can travel only a few centimetres in air. They can be absorbed by tissue paper or the outer layers of human skin (about 40 µm, equivalent to a few cells deep) and so are not generally dangerous to life unless the source is ingested or inhaled. Because of this high mass and strong absorption, however, if alpha radiation does enter the body through inhalation or ingestion, it is the most destructive form of ionizing radiation, and with large enough dosage, can cause all of the symptoms of radiation poisoning. It is estimated that chromosome damage from α particles is 100 times greater than that caused by an equivalent amount of other radiation. ANNUAL LIMIT ON The intake in to the body by inhalation, ingestion or through the skin of a INTAKE (ALI) given radionuclide in a year that would result in a committed dose equal to the relevant dose limit . -

Nuclear Fusion Enhances Cancer Cell Killing Efficacy in a Protontherapy Model
Nuclear fusion enhances cancer cell killing efficacy in a protontherapy model GAP Cirrone*, L Manti, D Margarone, L Giuffrida, A. Picciotto, G. Cuttone, G. Korn, V. Marchese, G. Milluzzo, G. Petringa, F. Perozziello, F. Romano, V. Scuderi * Corresponding author Abstract Protontherapy is hadrontherapy’s fastest-growing modality and a pillar in the battle against cancer. Hadrontherapy’s superiority lies in its inverted depth-dose profile, hence tumour-confined irradiation. Protons, however, lack distinct radiobiological advantages over photons or electrons. Higher LET (Linear Energy Transfer) 12C-ions can overcome cancer radioresistance: DNA lesion complexity increases with LET, resulting in efficient cell killing, i.e. higher Relative Biological Effectiveness (RBE). However, economic and radiobiological issues hamper 12C-ion clinical amenability. Thus, enhancing proton RBE is desirable. To this end, we exploited the p + 11Bà3a reaction to generate high-LET alpha particles with a clinical proton beam. To maximize the reaction rate, we used sodium borocaptate (BSH) with natural boron content. Boron-Neutron Capture Therapy (BNCT) uses 10B-enriched BSH for neutron irradiation-triggered alpha-particles. We recorded significantly increased cellular lethality and chromosome aberration complexity. A strategy combining protontherapy’s ballistic precision with the higher RBE promised by BNCT and 12C-ion therapy is thus demonstrated. 1 The urgent need for radical radiotherapy research to achieve improved tumour control in the context of reducing the risk of normal tissue toxicity and late-occurring sequelae, has driven the fast- growing development of cancer treatment by accelerated beams of charged particles (hadrontherapy) in recent decades (1). This appears to be particularly true for protontherapy, which has emerged as the most-rapidly expanding hadrontherapy approach, totalling over 100,000 patients treated thus far worldwide (2). -

ACUTE RADIATION SYNDROME: Diagnosis and Treatment
ACUTE RADIATION SYNDROME: Diagnosis and Treatment Badria Al Hatali, MD Medical Toxicologist Department of Environmental and Occupational Health MOH - Oman Objectives Provide a review of radiation basics and acute radiation sickness Discuss diagnostic tools and triage tools for Acute Radiation Syndromes Discuss management of Acute Radiation Syndromes Energy traveling over a distance as Waves Particles • Gamma rays • Alpha • X-rays • Beta • Radio waves • neurons Non-ionizing vs Ionizing Radiation • High energy • Low energy • Removes orbital electrons • Does not remove orbital from atoms > DNA electrons from atom damage Radioactive Decay Process to Remove excess energy from atomic nuclei Nuclei emit rays or particles to decrease nuclear energy Radioactive materials have unstable nuclei with excess energy Ionizing Radiation Dose • Radiation absorb dose (RAD): the amount of energy absorbed by the body. 1 cGy = 0.01 J/kg (USA) • Gray (Gy): expressed as absorbed energy per unit mass of tissue. 100 rad =100 cGy =1 J/kg (SI) • Roentgen Equivalent Man (REM) relates the absorbed dose in human tissue to the effective biological damage of the radiation (USA) • Sievert (Sv): the absorbed dose in human tissue to the effective biological damage of the radiation (SI) Radioactivity Biological And Effective Half-lives Biological half-life is the time to remove half of radioactive element from body Effective half-life is the combined effect of radioactive decay & biological elimination Effective half-life is always shorter than either physical or biological half-lives Biological Effects of Ionizing Radiation Direct damage Chromosome Other biochemical E.g. alpha and beta particles Indirect damage Chemical changes due to radiolysis of water in cell E.g. -

Emergency and Combat First Aid» Module № 1 Emergency and Combat First Aid Topic 7 Means of Mass Destruction
Ministry of Health of Ukraine Ukrainian Medical stomatological Academy It is ratified On meeting department Of accident aid and military medicine «___»_____________20 __y. Protocol №_____ Manager of department DMSc ., assistant professor __________К.Shepitko METHODICAL INSTRUCTION FOR INDEPENDENT WORK OF STUDENTS DURING PREPARATIONS FOR THE PRACTICAL LESSON Educational discipline «Emergency and Combat First Aid» Module № 1 Emergency and Combat First Aid Topic 7 Means of Mass Destruction. First Aid. Weapons of mass destructions. Lesson 10 Radiations chemical accidents .First Aid Сourse ІІ Foreing students training dentistry Faculty Training of specialists of the second (master) level of higher of education (название уровня высшего образования) Areas of knowledge _______ 22 «Health protection»_________ (шифр и название области знаний) Specialty ________222 «Medicine», 221 «Stomatology»________________ (код и наименование специальности) Poltava 2019 The relevance of the topic: Military action in modern warfare will be carried out with high activity and limit tension. They cause great losses in the army and among the population, the destruction of potentially dangerous objects, energy centers, waterworks, the formation of large zones of destruction, fires and floods. The main form of countering in the war, is armed struggle - the organized use of armed forces and weapons to achieve specific political and military objectives, a combination of military actions of varying scales. To conventional weapons, the application of which may cause losses among the population are missiles and aerial munitions, including precision munitions volumetric detonation of cluster and incendiary. Have the greatest efficiency high precision conventional weapons, which provide automatic detection and reliable destruction of targets and enemy targets with a single shot (trigger). -
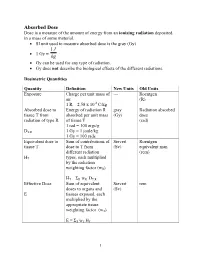
Absorbed Dose Dose Is a Measure of the Amount of Energy from an Ionizing Radiation Deposited in a Mass of Some Material
Absorbed Dose Dose is a measure of the amount of energy from an ionizing radiation deposited in a mass of some material. • SI unit used to measure absorbed dose is the gray (Gy). 1J • 1 Gy = kg • Gy can be used for any type of radiation. • Gy does not describe the biological effects of the different radiations. Dosimetric Quantities Quantity Definition New Units Old Units Exposure Charge per unit mass of --- Roentgen air (R) 1 R = 2.58 x 10-4 C/kg Absorbed dose to Energy of radiation R gray Radiation absorbed tissue T from absorbed per unit mass (Gy) dose radiation of type R of tissue T (rad) 1 rad = 100 ergs/g DT,R 1 Gy = 1 joule/kg 1 Gy = 100 rads Equivalent dose to Sum of contributions of Sievert Roentgen tissue T dose to T from (Sv) equivalent man different radiation (rem) HT types, each multiplied by the radiation weighting factor (wR) HT = ΣR wR DT,R Effective Dose Sum of equivalent Sievert rem doses to organs and (Sv) E tissues exposed, each multiplied by the appropriate tissue weighting factor (wT) E = ΣT wT HT 1 Radiological Protection For practical purposes of assessing and regulating the hazards of ionizing radiation to workers and the general population, weighting factors (previously called quality factors, Q) are used. A radiation weighting factor is an estimate of the effectiveness per unit dose of the given radiation relative a to low-LET standard. Weighting factors are dimensionless multiplicative factors used to convert physical dose (Gy) to equivalent dose (Sv) ; i.e., to place biological effects from exposure to different types of radiation on a common scale. -

Exposure Data
X-RADIATION AND γ-RADIATION 1. Exposure data 1.1 Occurrence 1.1.1 X-radiation X-rays are electromagnetic waves in the spectral range between the shortest ultraviolet (down to a few tens of electron volts) and γ-radiation (up to a few tens of mega electron volts) (see Figure 2, Overall introduction). The term γ-radiation is usually restricted to radiation originating from the atomic nucleus and from particle annihilation, while the term X-radiation covers photon emissions from electron shells. X-rays are emitted when charged particles are accelerated or decelerated, during transitions of electrons from the outer regions of the atomic shell to regions closer to the nucleus, and as bremsstrahlung, i.e. radiation produced when an electron collides with, or is deflected by, a positively charged nucleus. The resulting line spectra are characteristic for the corresponding element, whereas bremsstrahlung shows a conti- nuous spectrum with a steep border at the shortest wavelengths. Interaction of X-rays with matter is described by the Compton scattering and photoelectric effect and their resulting ionizing potentials, which lead to significant chemical and biological effects. Ions and radicals are produced in tissues from single photons and cause degradation and changes in covalent binding in macromolecules such as DNA. In other parts of the electromagnetic spectrum, below the spectra of ultraviolet and visible light, the single photon energies are too low to cause genotoxic × –μ⋅d effects. The intensity (I) of X-rays inside matter decreases according to I = I0 10 , where d is the depth and μ a coefficient specific to the interacting material and the corresponding wavelength. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Chapter 2: Dosimetricprinciples, Quantitiesandunits
Chapter 2: Dosimetric Principles, Quantities and Units Set of 131 slides based on the chapter authored by J.P. Seuntjens, W. Strydom, and K.R. Shortt of the IAEA publication (ISBN 92-0-107304-6): Review of Radiation Oncology Physics: A Handbook for Teachers and Students Objective: To familiarize the student with the basic principles of the quantities used in dosimetry for ionizing radiation. Slide set prepared in 2006 by G.H. Hartmann (Heidelberg, DKFZ) Comments to S. Vatnitsky: [email protected] Version 2012 IAEA International Atomic Energy Agency CHAPTER 2. TABLE OF CONTENTS 2.1 Introduction 2.2 Radiation field quantities (also denoted as Radiometric quantities) 2.3 Dosimetrical quantities: fundamentals 2.4 Dosimetrical quantities 2.5 Interaction coefficients: electrons 2.6 Interaction coefficients: photons 2.7 Relation between radiation field and dosimetric quantities 2.8 Cavity theory IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 2.Slide 1 2.1 INTRODUCTION Radiation dosimetry has its origin in the medical application of ionizing radiation starting with the discovery of x-rays by Röntgen in 1895. In particular • the need of protection against ionizing radiation, • the application in medicine required quantitative methods to determine a "dose of radiation". The purpose of a quantitative concept of a dose of radiation is: • to predict associated radiation effects (radiation detriments) • to reproduce clinical outcomes. IAEA Review of Radiation Oncology Physics: A Handbook for Teachers and Students - 2.1. Slide 1 2.1 INTRODUCTION The connection to the medical profession is obvious. The term dose of radiation was initially used in a pharmacological sense, that means: analogously to its meaning when used in prescribing a dose of medicine. -

1. the Three Types of Nuclear Radiation Are Protons, Electrons, Neutrons
Page 1 of 8 This chapter has 53 questions. Scroll down to see and select individual questions or 0 questions at random and keep in order narrow the list using the checkboxes below. Multiple Choice Questions - (45) Topic: Nuclear reactions and nuclear fission - (4) Fill In The Blank Questions - (8) Topic: Nuclear reactors - (11) Odd Numbered - (27) Topic: Nuclear weapons and nuclear fusion - (9) Even Numbered - (26) Topic: Radioactive decay - (19) Accessibility: Keyboard Navigation - (45) Topic: The structure of the nucleus - (10) Difficulty: Easy - (46) Type: Conceptual - (50) Difficulty: Hard - (1) Type: Definition - (10) Difficulty: Medium - (6) Type: Numerical - (3) 1. The three types of nuclear radiation are protons, electrons, neutrons. electrical, strong nuclear, weak nuclear. radiation, convection, conduction. → gamma, beta, alpha. Accessibility: Keyboard Navigation Difficulty: Easy Topic: Radioactive decay Multiple Choice Question Type: Conceptual MC The three types of nuclear radiation are Type: Definition 2. The word "radioactive" means an atomic nucleus absorbs neutrons. there is significant interference between atomic radiation and radio reception. → there are nuclei present which will spontaneously emit nuclear radiation. an atom spontaneously captures an electron from a neighboring atom. Accessibility: Keyboard Navigation Difficulty: Easy Multiple Choice Question Topic: Radioactive decay MC The word radioactive means Type: Conceptual 3. The discovery of the neutron helped people understand → how two atoms of the same element can have different atomic masses. why the nucleus has a positive charge. how electrons are attracted to the nucleus. why the nucleus is much more massive than the electrons in an atom. Accessibility: Keyboard Navigation Difficulty: Easy Multiple Choice Question Topic: The structure of the nucleus MC The discovery of the neutron helped people u.. -

The Potential Detrimental Impact of Galactic Cosmic Radiation on Central Nervous System and Hematopoietic Stem Cells
THE POTENTIAL DETRIMENTAL IMPACT OF GALACTIC COSMIC RADIATION ON CENTRAL NERVOUS SYSTEM AND HEMATOPOIETIC STEM CELLS By RUTULKUMAR UPENDRABHAI PATEL Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: Dr. Scott M. Welford, Ph.D Department of Pharmacology CASE WESTERN RESERVE UNIVERSITY January, 2019 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Rutulkumar Upendrabhai Patel Candidate for the Doctor of Philosophy degree *. (signed) Derek Taylor (Committee Chair) Scott M. Welford (Dissertation Advisor) Stanton L. Gerson (Committee Member) Marvin Nieman (Committee Member) Jennifer Yu (Committee Member) (date) December 3rd, 2018 *We also certify that written approval has been obtained for any proprietary material contained therein. ii Dedication I would like to dedicate this dissertation to my parents, Upendrabhai and Ujvalakumari Patel, who supported my wishes and ambitions despite being lived most of their lives in a lower-middle class family income. They sacrificed a lot to make sure a better life for their children. I would also like to dedicate this to my two sisters, Ekta and Vanita, for their support and encouragement over the years. iii Table of Contents Table of Contents ……………………………………………………………….. iv List of Figures ………………………………………………………………….. viii Acknowledgements ……………………………………………………………. xii Abstract ……………………………………………………………….…………. 1 Chapter 1: Introduction and Background ………………………………….. 3 1.1 Radiation, DNA Damage, and Carcinogenesis …………………………... 3 1.1.1 Space Radiation Environment and Induction of DNA Damage …………………………………………………………… 8 1.1.2 Radiation Induced Carcinogenesis ……………….................... 10 1.2 Hematopoietic Stem Cell Niche and Functions …………………………… 12 1.2.1 Low-LET Irradiation and HSC Injuries …………………………. 16 1.2.2 High-LET Irradiation Impact on HSCs ………………………….