The Life Science Valley in the Heart of Europe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

923.250 Decreto Esecutivo Concernente Le Zone Di Protezione Pesca 2019-2024
923.250 Decreto esecutivo concernente le zone di protezione pesca 2019-2024 (del 24 ottobre 2018) IL CONSIGLIO DI STATO DELLA REPUBBLICA E CANTONE TICINO visti – la legge federale sulla pesca del 21 giugno 1991 (LFSP) e la relativa ordinanza del 24 novembre 1993 (OLFP), – la legge cantonale sulla pesca e sulla protezione dei pesci e dei gamberi indigeni del 26 giugno 1996 e il relativo regolamento di applicazione del 15 ottobre 1996, in particolare l’art. 19, – la Convenzione tra la Confederazione Svizzera e la Repubblica Italiana per la pesca nelle acque italo-svizzere del 1° aprile 1989, in particolare l’art. 6, decreta: Art. 1 Per il periodo dal 1° gennaio 2019 al 31 dicembre 2024, sono istituite le seguenti zone di protezione. a) Zone di protezione permanenti nei corsi d’acqua, dove la pesca è vietata: 1. Riale di Golino: dalla confluenza con il fiume Melezza alla prima cascata a monte della strada cantonale. 2. Torrente Brima ad Arcegno: dalla confluenza con il riale «Mulin di Cioss» all’entrata del paese di Arcegno, nonché tutta la tratta a valle dello sbarramento presso i mulini Simona a Losone. 3. Torrente Ribo a Vergeletto: dal ponte in località Custiell (pto. 891, poco a monte di Vergeletto) al ponte in ferro in località Zardin. 4. Fiume Bavona a Bignasco-Cavergno: dal ponte della cantonale a Bignasco fino alla passerella di Cavergno. 5. Roggia della piscicoltura di Sonogno: tutto il corso d’acqua fino alla confluenza con il fiume Verzasca. 6. Ronge di Alnasca a Brione Verzasca: dalla confluenza con il fiume Verzasca alle sorgenti. -

Comunicato Stampa L'istituto Scolastico Di Lugano Attiva Il Servizio Di Accudimento
P. 1 di 2 Città di Lugano Lugano, 18 marzo 2020 Comunicazione e relazioni istituzionali Ufficio stampa e PR Piazza della Riforma 1 6900 Lugano Svizzera t. +41 58 866 70 88 [email protected] www.lugano.ch Comunicato stampa L’Istituto scolastico di Lugano attiva il servizio di accudimento COVID-19 L’Istituto scolastico di Lugano garantirà l’accoglienza degli allievi della scuola dell’infanzia e della scuola elementare che non possono rimanere a casa. Come noto, a seguito delle disposizioni federali per limitare la diffusione da nuovo Coronavirus, la chiusura delle scuole è stata prorogata fino al 19 aprile e le lezioni sospese. I figli di genitori che possono accudire o far accudire i propri figli senza scambi intergenerazionali (persone anziane o con malattie croniche come da indicazione cantonale) devono stare a casa. L’Istituto scolastico di Lugano è a disposizione - durante il normale orario scolastico - per accudire gli allievi della scuola dell’infanzia e della scuola elementare che non possono rimanere a casa. Considerato il numero limitato di bambini che hanno sino ad ora usufruito del servizio, si informa che l’accoglienza nelle rispettive sedi di scolarizzazione verrà garantita fino a oggi, mercoledì 18 marzo 2020. A partire da lunedì 23 marzo, gli allievi che per comprovati motivi necessitano dell’accudimento da parte delle scuole saranno ospitati nelle sedi indicate di seguito. Gli allievi delle scuole dell’infanzia di: . Cadro, Bozzoreda, Davesco-Soragno, Dino 1, Dino 2, Sonvico, Maglio di Colla, Piccolo Mondo, Terzerina, Villa Luganese verranno accuditi presso la SI Terzerina, via Terzerina a Pregassona. Brè, Cassarate, Ruvigliana, Viganello-Albonago, Via Bottogno-Viganello presso la SI Via Bottogno, via Bottogno a Viganello. -
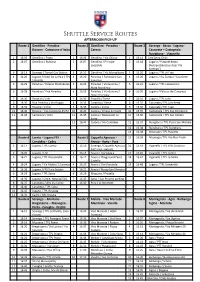
Afternoon Drop-Off 2021-2022
Shuttle Service Routes AFTERNOON PICK-UP Route 1 Gentilino - Paradiso - Route 2 Gentilino - Paradiso - Route 3 Sorengo - Besso - Lugano - Bissone - Campione d’Italia Carona Cassarate – Castagnola - Ruvigliana - Viganello 1 16.05 Gentilino / Posta 1 16.06 Gentilino / Via Chioso 1 16.14 Sant’Anna Clinic 2 16.07 Gentilino / Rubiana 2 16.07 Gentilino / Principe 2 16.18 Lugano / Piazzale Besso Leopoldo (Autopostale) bus stop ‘Via Sorengo’) 3 16.13 Sorengo / Tamoil Gas Station 3 16.10 Gentilino / Via Montalbano 3 16.20 Lugano / TPL Ai Frati 4 16.20 Lugano / Hotel De La Paix / TPL 4 16.20 Paradiso / Funicolare San 4 16.20 Lugano / Via Zurigo / ‘Casaforte’ S.Birgitta Salvatore 5 16.25 Paradiso / Palazzo Mantegazza 5 16.22 Paradiso / Via Guidino / 5 16:22 Lugano / TPL Cappuccine Nizza Residence 6 16.28 Paradiso / Riva Paradiso 6 16.23 Paradiso / Via Guidino / 6 16.30 Lugano / Palazzo dei Congressi Hotel The View 7 16.30 Paradiso / Lido 7 16.30 Pazzallo / Paese 7 16:31 Lugano / TPL Lido 8 16.30 Riva Paradiso / Via Boggia 8 16.33 Carabbia / Paese 8 16.32 Cassarate / TPL Lanchetta 9 16.35 Bissone / Circle 9 16.35 Carona / Ciona 9 16.33 Cassarate / TPL Lago 10 16.40 Bissone / Via Campione 45/55 10 16.40 Carona / Chiesa dei Santi 10 16.35 Castagnola / TPL San Domenico 11 16.43 Campione / Arco 11 16.45 Carona / Restaurant La 11 16.36 Castagnola / TPL San Giorgio Sosta 12 16:47 Carona / Via Colombei 12 16.37 Ruvigliana / TPL Parco San Michele 13 16.38 Ruvigliana / TPL Suvigliana 14 16:38 Albonago / TPL Ruscello Route 4 Loreto - Lugano FFS - Route 5 Cappella -
Quartiere Di Pambio – Noranco
DISPOSIZIONI RIFIUTI 2019 CITTÀ DI LUGANO DOVE DEPOSITARE I RIFIUTI QUARTIERE DI PAMBIO – NORANCO PIEGA TIPOLOGIA DI RIFIUTI, GIORNI E LUOGHI DI RACCOLTA RSU CARTA E CARTONE VETRO I rifiuti solidi urbani (sacchi delle economie domestiche) La carta può essere depositata negli appositi contenitori I vuoti in vetro (esclusi specchi, ceramiche, finestre, vetri vengono raccolti 2 volte la settimana - martedì e venerdì situati presso l’ecopunto Bagion, come pure sulla Strada da d’auto, lampadine) vanno sciacquati, separati dalle parti - esclusi i giorni festivi. I sacchi sono da depositare fra Igia oppure consegnata all’ecocentro. Il cartone, specie se di materiale diverso (coperchi, tappi, ...) e depositati nei le 18:00 della sera precedente la raccolta e le 6:00 del piegato e non tagliato in pezzi di misura adeguata, non va diversi contenitori a disposizione sul territorio comunale, giorno prestabilito, negli appositi cassonetti. In alcune inserito nei contenitori interrati, ove rischia di incastrarsi; da lunedì a sabato, tra le 8:30 e le 20:00 (domenica e zone sono già in funzione o entreranno in funzione dei deve essere consegnato all’ecocentro. giorni festivi esclusi). Vi sono appositi contenitori situati contenitori interrati, a disposizione 24 ore su 24, tutti i all’ecopunto Bagion, come pure sulla Strada da Igia, sulla giorni della settimana. Strada dal Brugòn e sulla Strada da la Curona. È possibile la consegna all’ecocentro. SCARTI VEGETALI Gli scarti vegetali provenienti da lavori di giardinaggio INGOMBRANTI effettuati dai privati cittadini possono essere consegnati ALLUMINIO E LATTA Per ingombranti s’intendono solo i rifiuti di grandi di- all’ecocentro. -

Ticino 201 Ä a Castione–Bellinzona, Stazione– Camorino (Vedi 62.201
Riproduzione Reproduction Gewerbliches www.fahrplanfelder.ch 2015 62.000 2378 Linie/Ligne Linie/Linea T ranvia = T Ticino commerciale commerciale Reproduzieren O = Filobus 201 ä A Castione–Bellinzona, Stazione– A = Autobus Camorino (vedi 62.201) 202 ä A Bellinzona, Stazione–Monte Carasso– Sementina–Giubiasco (vedi 62.202) interdite vietato 203 ä A Bellinzona, Stazione–Giubiasco– Camorino–S. Antonino (vedi 62.203) verboten 204 ä A Bellinzona, Stazione–Artore–Cast. Sasso Corbaro (vedi 62.204) 205 ä A Via Gerretta– Bellinzona, Stazione– Ospedale (Ravecchia) (vedi 62.205) APB Città/AutoPostale Svizzera SA (PAG), Regione Ticino & 0900 311 311 (CHF 1.19/min da rete fissa CH) 1 ä A Tenero–Minusio–Locarno, Stazione– Solduno–Ascona, Posta (vedi 62.301) 2 ä A Locarno–Monti della Trinità–Orselina Brione s. Minusio (vedi 62.302) 5 ä A Ascona, Sonnenhof–Ascona, Monte Verità (Linea 5) 7 ä A Locarno, Stazione–Losone, Posta– Arbigo–Zandone (vedi 62.307) 8 ä A Collina di Brissago (vedi 62.308) FART & 091 756 04 40, www.centovalli.ch 1 ä A Lugano, Centro–Paradiso–P+R Fornaci (vedi 62.401) 2 ä A Paradiso–Loreto–Lugano, Stazione– Lugano, Centro–Cassarate–Castagnola (vedi 62.402) 3 ä A Pregassona–Molino Nuovo– Lugano, Centro–Besso–Breganzona (vedi 62.403) 4 ä A Lugano, Centro–Loreto– Lugano, Stazione–Cornaredo–Trevano– Canobbio (vedi 62.404) 5 ä A Vezia–Crocifisso–Massagno– Lugano, Centro–Viganello (vedi 62.405) 8 ä A Paradiso–Pambio–Noranco–Paradiso Paradiso–Pazzallo–Carabbia–Senago– Paradiso (vedi 62.408) 9 ä A Viganello–Pregassona–Cureggia (vedi 62.409) -

Analisi Annuali 2019 Dell'acqua Potabile Distribuita Dalle AIL SA
Analisi annuali 2019 dell’acqua potabile distribuita dalle AIL SA 125° acquedoo di Lugano 1894-2019 Era il 23 dicembre 1894 quando Lugano festeggiò l’arrivo dell’acqua potabile, un evento storico che avrebbe dato un contributo indispensabile allo sviluppo della Città e un’opera destinata a divenirne una pietra miliare: un sistema di condot - te per sfruttare le sorgenti dei monti Gradiccioli e Tamaro e portare l’acqua fino a Lugano. Da quel primo importante passo l’acquedotto di Lugano non ha mai smesso di svilupparsi e rinnovarsi per stare al passo con la crescita della popolazione e le esigenze di una città in espansione. Oggi come allora la nostra passione è immutata, così come il nostro impegno a fornire ai cittadini un’acqua di qualità: buo - na, economica, ecologica. Settore approvvigionamento Settore approvvigionamento Lugano Integrata Anno 2019 Lugano Caprino Anno 2019 Bacino Bacino Comune di Lugano, quartieri di: Barbengo, Besso, Bré-Aldesago, Bre - Comune di Lugano, quartiere di Castagnola zona Caprino ganzona, Cadro, Carabbia, Carona, Castagnola-Cassarate-Ruvigliana, Cu - reggia, Davesco-Soragno, Gandria, Loreto, Lugano Centro, Molino Valutazione generale Nuovo, Pambio Noranco, Pregassona, Sonvico, Viganello, Villa Luganese Qualità "Classe OMS" anno 2018 eccellente e Comune di Massagno. Potabilità anno 2018 1 avviso di non potabilità Durezza dolce Valutazione generale Caratteristiche chimiche equilibrio Qualità "Classe OMS" anno 2018 eccellente Mineralizzazione debolmente mineralizzata Potabilità anno 2018 1 avviso di non potabilità -

Linee Trasporti Pubblici Luganesi SA Valide a Partire Dal 13.12.2020
Linee Trasporti Pubblici Luganesi SA Valide a partire dal 13.12.2020 Giubiasco Bellinzona Origlio- Sureggio- Carnago- Manno- Piano Stampa Locarno Tesserete Tesserete Tesserete Bioggio Capolinea Basilea-Zurigo a Lamone Farer Cadempino Comano Canobbio Stazione Ganna lio Mag te on le P al V Manno di Uovo di Manno 19 Cadempino Municipio Canobbio Cadempino Comano Ronchetto Mercato Resega Studio TV Cureglia Rotonda Comano cio Rotonda c de Resega er Ghia Trevano ta ce V is o Centro Studi P Cr Villa 7 Negroni 19 a ic Resega Term Vezia Paese o Lugano Cornaredo ared i/Corn an i Stadio Est a Ci Cureggia Vi tan Pregassona ren Paese ia B Piazza di Giro Vignola V na ami tr Villa Recreatio Bel Cà Rezzonico a Vi a Viganello za S. Siro lla an sa Seren Vi st Ospedale Vignola Scuole Ca Co Civico Molino Nuovo 2 ovo h i sc ch Scuole a Nu tà ri n z o si F Piazza Molino Nuovo az in er x Ro o Pi l v a sc Praccio Molino Nuovo 2 i ai Mo Un ia M Bo V al al Sole Sassa Via Santa Lucia Zurigo Sacro ta Brè Pianazzo Vicolo Cuore San vecchio a le Paese Paese l Albonago o Autosilo ia edano ag ez ia Balestra Corsolv Ospal Paese ldes Vergiò E It A Genzana - to u o A o a i que Vie l in Si C io Via Balestr Rad Gradinata S. Anton udio Lugano St lao Suvigliana co i Centro o t Scuole Funicolare di ri Ni Cassarate to S. -

M U N I C I P I O M U N I C I P I O MESSAGGIO MUNICIPALE NO. 9317 Piano Regolatore Unitario Di Lugano. Elaborazione Del Masterp
M U N I C I P I O M U N I C I P I O MESSAGGIO MUNICIPALE NO. 9317 Piano Regolatore unitario di Lugano. Elaborazione del Masterplan ai sensi degli artt. 18 cpv.2 Lst e 25 RLst. Richiesta di un credito di Fr. 1'414'000 per l’elaborazione del Masterplan e per l’informazione pubblica. Lugano, 1° ottobre 2015 All'On.do Consiglio Comunale 6900 Lugano Onorevole Signor Presidente, Onorevoli Signori Consiglieri Comunali, sottoponiamo al vostro esame e alla vostra approvazione il presente Messaggio Municipale con il quale si chiede l’avvio delle procedure necessarie all’elaborazione dello Studio di base (Masterplan) del nuovo Piano Regolatore unitario di Lugano, nonché la richiesta di un credito di Fr. 1'414'000 per sostenerne i costi fino all’elaborazione del documento definitivo e per lo svolgimento dell’informazione pubblica. - 1 - 1. PREMESSA E CRONISTORIA Tra le sfide poste alla Nuova Lugano in seguito alle ultime fasi aggregative, vi è indubbiamente quella di dotare la Città di uno strumento pianificatorio unitario e coordinato per l’insieme del territorio comunale , rispettivamente quella di affrontare, in questo contesto, l’ aggiornamento del PR di Lugano, Castagnola e Brè e dei PR dei Quartieri aggregati di più vecchia generazione. Già nel 2000, in accoglimento della mozione del 1° aprile 1999 dell’on. Simonetta Perucchi-Borsa , il Legislativo comunale aveva espresso una serie di considerazioni ed indicazioni, sottolineando in particolare la necessità di un nuovo ordinamento pianificatorio che non fosse strettamente contenuto nei limiti giurisdizionali del Comune, ma che considerasse un ambito territoriale più ampio. -

Sezione Di Brè. Elaborazione Del Piano Di Indirizzo Ai Sensi Degli Artt
M U N I C I P I O MESSAGGIO MUNICIPALE NO. 9318 Revisione Piano Regolatore di Lugano - Sezione di Brè. Elaborazione del Piano di Indirizzo ai sensi degli artt. 18 cpv.2 Lst e 25 RLst. Richiesta di un credito di Fr. 286'200 per l’elaborazione del Piano di Indirizzo e per l’informazione pubblica concernente la Revisione del Piano Regolatore della Sezione di Brè Lugano, 1° ottobre 2015 All'On.do Consiglio Comunale 6900 Lugano Onorevole Signor Presidente, Onorevoli Signori Consiglieri Comunali, sottoponiamo al vostro esame e alla vostra approvazione il presente Messaggio Municipale con il quale si chiede l’avvio delle procedure necessarie all’elaborazione del Piano di Indirizzo per la Revisione del Piano Regolatore della Sezione di Brè, nonché la richiesta di un credito di Fr. 286’200 per sostenerne i costi fino all’elaborazione del documento definitivo e per lo svolgimento dell’informazione pubblica. 1. PREMESSA E CRONISTORIA 1.1 L’accoglimento della Mozione no. 3616 “Il PR di Brè è da rivedere subito” Il Consiglio Comunale nella sua seduta del 2 febbraio 2015 ha accolto parzialmente la Mozione no. 3616 del 14 novembre 2010, degli On.li Zanini Barzaghi (PS), Badaracco (PLR), Cattaneo (Verdi), Corti (PS), Degiorgi (PS), Endriss (PPD), Ghisletta (PS), Gilardi (LdT), Jalkanen Keller (Verdi), Macchi (PLR), Perucchi Borsa (PPD), Rossi (PS) e Tarchini (PPD), dal titolo "Il Piano Regolatore di Brè è da rivedere subito". Tra le richieste del Consiglio Comunale, alle quali il Municipio dovrà cercare di dare seguito vi è una revisione generale del Piano Regolatore relativo al quartiere di Brè, in particolare per: 1 a. -

8047 Vella Bv
190537 Com Paradiso Cop:190537 Com Paradiso Cop 29.1.2010 15:58 Pagina 1 Comune di Paradiso Guida per conoscere meglio il nostro Comune 190537 Com Paradiso Cop:190537 Com Paradiso Cop 29.1.2010 15:58 Pagina 2 Scegliete chi sa scegliere. Direzione Generale e Agenzia di città Abbiamo scelto la trasparenza, Via Giacomo Luvini 2a, CH–6900 Lugano la prudenza, la qualità del servizio. Tel. +41 58 855 32 00 Fate anche voi la scelta giusta: scegliete BPS (SUISSE). Sede Principale Anche in tempi difficili. Via Maggio 1, CH–6900 Lugano Tel. +41 58 855 31 00 Succursali ed Agenzie Call Center 00800 800 767 76 Chiasso, Mendrisio, Lugano-Cassarate, Bellinzona, Biasca, Locarno www.bps-suisse.ch Prossima apertura prevista per autunno 2009 Banca Popolare di Sondrio (SUISSE) Paradiso (Palazzo Mantegazza) La Banca che parla con te. 190537 Com Paradiso Int:190537 Impa Paradiso 29.1.2010 16:02 Pagina 1 Foto di Bruno Pellandini 190537 Com Paradiso Int:190537 Impa Paradiso 29.1.2010 16:02 Pagina 2 190537 Com Paradiso Int:190537 Impa Paradiso 29.1.2010 16:02 Pagina 3 SALUTO DEL SINDACO Bonum Nomen, Bonum Omen! Con questo significativo auspicio – buon nome, e sull'organizzazione sociopolitica, amministrativa buon augurio – colgo lo spunto per porgere a tutti e culturale della comunità di Paradiso. i nuovi arrivati, ospiti e cittadini, il più cordiale e sincero benvenuto nel nostro bel Paradiso. Il servizio ai Cittadini e il dialogo, e quindi una mi- gliore trasparenza, è uno degli obiettivi della no- Paradiso era un tempo chiamata Calprino e prende stra Amministrazione. -

0.818.694.54 Accordo Tra Il Governo Svizzero E Il Governo Italiano Concernente La Traslazione Di Salme Nel Traffico Locale Di Confine
Testo originale 0.818.694.54 Accordo tra il Governo svizzero e il Governo italiano concernente la traslazione di salme nel traffico locale di confine Tra la Legazione di Svizzera a Roma e il Ministero italiano degli affari esteri ha avuto luogo in data 11 aprile/14 maggio 1951 uno scambio di note relativo alla traslazione di salme nel traffico locale di confine. La nota svizzera è del seguente tenore: 1. La Convenzione internazionale concernente il trasporto dei cadaveri con- chiusa a Berlino il 10 febbraio 19371 alla quale hanno aderito tanto l’Italia quanto la Svizzera, prevede all’articolo 10 che le sue disposizioni non si ap- plicano al trasporto dei cadaveri nel traffico locale di confine. I due Governi decidono di adottare in proposito la seguente procedura: 2. Il traffico locale di confine comprende nella fattispecie una zona sita dall’una e dall’altra parte della frontiera italo-svizzera che si estende per circa 10 km dalla frontiera stessa; la procedura per il trasporto dei cadaveri, nell’ambito delle due zone, si applica alle località comprese negli elenchi allegati. 3. I lascia-passare per cadaveri sono rilasciati, da parte svizzera, dalle autorità cantonali e, da parte italiana, dalle autorità provinciali. In Svizzera le autorità competenti sono, per il Cantone dei Grigioni, i com- missariati distrettuali, per il Canton Ticino, le autorità comunali e per il Canton Vallese, la polizia cantonale. In Italia le autorità competenti sono i Prefetti delle Provincie di Como, Va- rese, Sondrio, Novara e il Commissario di Governo della Regione Trentino- Alto Adige2. 4. I lascia-passare per cadaveri non dovranno più essere muniti dell’autentica- zione delle firme. -

Foglioufficiale 102 2017
Si pubblica Repubblica e Bellinzona il martedì e il venerdì Cantone Ticino Venerdì 102 22 dicembre 2017 www.ti.ch/fu Anno 174 2017 n il le Cancelleria i co ficia Ogg o uf dello Stato ettin Boll i legg delle Foglio ufficial e U Atti legislativi e dell’Amministrazione B 11189 1 Atti dello stato civile – 2 Atti ed avvisi giudiziari 11222 3 Atti ed avvisi d’esecuzioni e fallimenti 11232 4 Atti ed avvisi comunali, patriziali, parrocchiali e consortili 11239 5 Atti diversi 11248 6 Iscrizioni nel Registro di commercio 11253 7 Gli avvisi per la parte interna Gli annunci pubblicitari Richieste amministrative e abbonamenti devono pervenire al più tardi alle ore 11 :00 sono da indirizzare esclusivamente a da inoltrare a di lunedì o giovedì all’indirizzo e-mail Publicitas SA Cancelleria dello Stato [email protected] Via Senago 42, 6915 Pambio Noranco Servizio di Segreteria del Consiglio di Stato telefono gratuito 0800 144 349 telefono 091 910 35 65 Amministrazione del Foglio ufficiale Tariffe fax 091 910 35 49 Piazza Governo 6, 6501 Bellinzona Linea di 1 mm di altezza e 103 di larghezza e-mail: [email protected] telefono 091 814 43 49 Avvisi ufficiali fr. 1.55 (+IVA 8% fr. 1.67 x mm) Tariffe fax 091 814 44 01 Avvisi diversi fr. 1.95 (+IVA 8% fr. 2.10 x mm) 1/1 pagina fr. 795.– / a colori fr. 950. – e-mail: [email protected] 1/2 pagina fr. 415.– / a colori fr. 515. – Tariffe e abbonamenti 1/4 pagina fr.