Chapter 1 Zoom in On… Patch Configurations in the Jargon of Electrophysiologists, a Patch Is a Piece of Neuronal Membrane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nernst Potentials and Membrane Potential Changes
UNDERSTANDING MEMBRANE POTENTIAL CHANGES IN TERMS OF NERNST POTENTIALS: For seeing how a change in conductance to ions affects the membrane potential, follow these steps: 1. Make a graph with membrane potential on the vertical axis (-100 to +55) and time on the horizontal axis. 2. Draw dashed lines indicating the standard Nernst potential (equilibrium potential) for each ion: Na+ = +55 mV, K+ = -90mV, Cl- = -65 mV. 3. Draw lines below the horizontal axis showing the increased conductance to individual ions. 4. Start plotting the membrane potential on the left. Most graphs will start at resting potential (-70 mV) 5. When current injection (Stim) is present, move the membrane potential upward to Firing threshold. 6. For the time during which membrane conductance to a particular ion increases, move the membrane potential toward the Nernst potential for that ion. 7. During the time when conductance to a particular ion decreases, move the membrane potential away from the Nernst potential of that ion, toward a position which averages the conductances of the other ions. 8. When conductances return to their original value, membrane potential will go to its starting value. +55mV ACTION POTENTIAL SYNAPTIC POTENTIALS 0mV Membrane potential Firing threshold Firing threshold -65mV -90mV Time Time Na+ K+ Conductances Stim CHANGES IN MEMBRANE POTENTIAL ALLOW NEURONS TO COMMUNICATE The membrane potential of a neuron can be measured with an intracellular electrode. This 1 provides a measurement of the voltage difference between the inside of the cell and the outside. When there is no external input, the membrane potential will usually remain at a value called the resting potential. -

Effect of Hyperkalemia on Membrane Potential: Depolarization
❖ CASE 3 A 6-year-old boy is brought to the family physician after his parents noticed that he had difficulty moving his arms and legs after a soccer game. About 10 minutes after leaving the field, the boy became so weak that he could not stand for about 30 minutes. Questioning revealed that he had complained of weakness after eating bananas, had frequent muscle spasms, and occasionally had myotonia, which was expressed as difficulty in releasing his grip or diffi- culty opening his eyes after squinting into the sun. After a thorough physical examination, the boy was diagnosed with hyperkalemic periodic paralysis. The family was advised to feed the boy carbohydrate-rich, low-potassium foods, give him glucose-containing drinks during attacks, and have him avoid strenuous exercise and fasting. ◆ What is the effect of hyperkalemia on cell membrane potential? ◆ What is responsible for the repolarizing phase of an action potential? ◆ What is the effect of prolonged depolarization on the skeletal muscle Na+ channel? 32 CASE FILES: PHYSIOLOGY ANSWERS TO CASE 3: ACTION POTENTIAL Summary: A 6-year-old boy who experiences profound weakness after exer- cise is diagnosed with hyperkalemic periodic paralysis. ◆ Effect of hyperkalemia on membrane potential: Depolarization. ◆ Repolarization mechanisms: Activation of voltage-gated K+ conductance and inactivation of Na+ conductance. ◆ Effect of prolonged depolarization: Inactivation of Na+ channels. CLINICAL CORRELATION Hyperkalemic periodic paralysis (HyperPP) is a dominant inherited trait caused by a mutation in the α subunit of the skeletal muscle Na+ channel. It occurs in approximately 1 in 100,000 people and is more common and more severe in males. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -
The Action Potential
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/6316219 The action potential ARTICLE in PRACTICAL NEUROLOGY · JULY 2007 Source: PubMed CITATIONS READS 16 64 2 AUTHORS, INCLUDING: Mark W Barnett The University of Edinburgh 21 PUBLICATIONS 661 CITATIONS SEE PROFILE Available from: Mark W Barnett Retrieved on: 24 October 2015 192 Practical Neurology HOW TO UNDERSTAND IT Pract Neurol 2007; 7: 192–197 The action potential Mark W Barnett, Philip M Larkman t is over 60 years since Hodgkin and called ion channels that form the permeation Huxley1 made the first direct recording of pathways across the neuronal membrane. the electrical changes across the neuro- Although the first electrophysiological nal membrane that mediate the action recordings from individual ion channels were I 2 potential. Using an electrode placed inside a not made until the mid 1970s, Hodgkin and squid giant axon they were able to measure a Huxley predicted many of the properties now transmembrane potential of around 260 mV known to be key components of their inside relative to outside, under resting function: ion selectivity, the electrical basis conditions (this is called the resting mem- of voltage-sensitivity and, importantly, a brane potential). The action potential is a mechanism for quickly closing down the transient (,1 millisecond) reversal in the permeability pathways to ensure that the polarity of this transmembrane potential action potential only moves along the axon in which then moves from its point -

Membrane Potentials As Signals
Membrane Potentials as Signals The membrane potential allows a cell to function as a battery, providing electrical power to activities within the cell and between cells. KEY POINTS A membrane potential is the difference in electrical potential between the interior and the exterior of a biological cell. In electrically excitable cells, changes in membrane potential are used for transmitting signals within the cell. The opening and closing of ion channels can induce changes from the resting potential. Depolarization is when the interior voltage becomes more positive and hyperpolarization when it becomes more negative. TERMS Graded potentials Graded potentials vary in size and arise from the summation of the individual actions of ligand- gated ion channel proteins, and decrease over time and space. Depolarization Depolarization is a change in a cell's membrane potential, making it more positive, or less negative, and may result in generation of an action potential. Action potential A short term change in the electrical potential that travels along a cell such as a nerve or muscle fiber. EXAMPLE In neurons, a sufficiently large depolarization can evoke an action potential in which the membrane potential changes rapidly. Give us feedback on this content: Membrane potential (also transmembrane potential or membrane voltage) is the difference in electrical potential between the interior and the exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions. -

1 Probing the Membrane Potential of Living Cells by Dielectric
Probing the membrane potential of living cells by dielectric spectroscopy Corina Bot and Camelia Prodan Dept. of Physics, New Jersey Inst. of Techn, Newark, NJ Abstract. In this paper we demonstrate a quantitative way to measure the membrane potential of live cells by dielectric spectroscopy. We also show that the values of the membrane potential obtained using our technique are in good agreement with those obtained using traditional methods-voltage sensitive dyes. The membrane potential is determined by fitting the experimental dielectric dispersion curves with the dispersion curves obtain from a theoretical model. Variations in the membrane potential were induced by modifying the concentration of potassium chloride in the solution of the cell suspension in the presence of valinomycin. For exemplification of the method, E. coli were chosen for our experiments. Introduction The resting membrane potential is one of the most important living cell parameters. By monitoring a cell’s membrane potential, one can get important information about its state. Changes in the membrane potential have been linked to different diseases, including Parkinson’s, epilepsy and Bartter’s syndrome [1]. A decrease in the plasma membrane potential has been seen in tumor cells with increased expression of multi-drug resistance (MDR) protein, also known as P-glycoprotein [2]. It was also shown that the membrane potential plays a critical role in the antibiotic uptake by cells and in bactericidal action [3]. Thus measuring or monitoring the membrane potential is of great importance. The existing “gold” or standard methods to measure or monitor the membrane potential are patch clamping and voltage sensitive dyes. -
Resting Potential and Chloride Channels
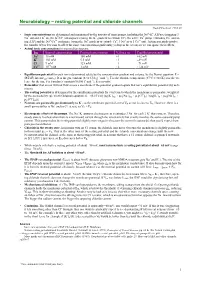
Neurobiology – resting potential and chloride channels Rudolf Cardinal, 4 Feb 99 • Ionic concentrations are determined and maintained by the activity of ionic pumps, including the Na+–K+ ATPase (pumping 3 + + + – + – + + Na out and 2 K in), the K /Cl cotransporter (using the K gradient to extrude Cl ), the active Ca pump (extruding Ca and us- ing ATP) and the Na+/Ca2+ exchanger (using the Na+ gradient to extrude Ca+, 3 Na+ in to 1 Ca2+ out). Action potentials involve the transfer of too few ions to affect the ionic concentration significantly (a drop in the ocean) so we can ignore their effects. • Actual ionic concentrations in mammalian neurons: Ion Internal concentration External concentration Valence (z) Equilibrium potential Na+ 15 mM 150 mM +1 +62 mV K+ 150 mM 5.5 mM +1 –89 mV Cl– 9 mM 125 mM –1 –71 mV Ca2+ 10–4 mM 1 mM +2 +124 mV • Equilibrium potential for each ion is determined solely by the concentration gradient and valence, by the Nernst equation: E = –1 –1 (RT/zF) ln(concout/concin). R is the gas constant (8.314 J deg mol ); T is the absolute temperature (37°C = 310 K); z is the va- lence for the ion; F is Faraday’s constant (96500 C mol–1); E is in volts. • Remember that an ion will not flow across a membrane if the potential gradient equals that ion’s equilibrium potential (by defi- nition). • The resting potential is determined by the equilibrium potentials for every ion to which the membrane is permeable, weighted + + – + + by the permeability (p), via th Goldman equation: E = (RT/F) ln[ (pK[K ]out + pNa[Na ]out + pCl[Cl ]in) / (pK[K ]in + pNa[Na ]in + – pCl[Cl ]out)]. -

The Cells That Make Us Who We Are How Neurons Communicate With
Communication within the Nervous System The Cells that make us who we are How neurons communicate with one another Garrett: Brain & Behavior 4e 1 The Cells That Make Us Who We Are • How many are there? • Neurons: 100 billion • Make up 10% of brain volume • Glia: Many more! • Make up 90% of brain volume • Neurons: Jobs include • convey sensory information to the brain; • carry out operations involved in thought and feeling; • Send commands out to the body. • Dendrites • Cell body or soma Garrett: Brain & Behavior 4e • Axons insulated with myelin (secreted by glia), with end terminals that release neurotransmitters from vesicles into the synapse 2 The Cells That Make Us Who We Are Figure 2.3: Components of a Neuron Garrett: Brain & Behavior 4e 3 The Cells That Make Us Who We Are Figure 2.4 a,b : The Three Shapes of Neurons • Unipolar neurons (a) • Bipolar neurons (b) • Multipolar neurons • Figure 2 .3, previous slide Garrett: Brain & Behavior 4e 4 The Cells That Make Us Who We Are Table 2.1: The Three Types of Neurons Figure 2.4c: The Three Shapes of Neurons Type Shape Description Motor neuron Multipolar Output to muscles/organs Sensory neuron Unipolar or Bipolar Input from receptors Interneuron Multipolar Most within the CNS. Most common. Garrett: Brain & Behavior 4e 5 The Cells That Make Us Who We Are Figure 2.5: Composition of the Cell Membrane • Lipids • Heads attracted to water in and outside the cell, tails repelled by water • Creates a double-layer membrane • Proteins • Hold the cells together • Controls the environment in and around the cell Garrett: Brain & Behavior 4e 6 The Neural Membrane • The neuron has a selectively-permeable membrane. -

NEURON and the PHYSIOLOGY of NERVE IMPULSE the Nervous
NEURON AND THE PHYSIOLOGY OF NERVE IMPULSE The nervous system is made of millions of structural and functional units called nerve cell or neurons and a number of supporting cells called glial cells. The function of the nerve cell is to receive and transmit impulses or messages and integrate the entire body as a single unit. The neurons can be classified into three types – 1. Unipolar neuron- it has a cell body or cyton from which arises a single short branch called neurite that immediately divides into the dendritic or receiving branch and axonic or transmitting branch. 2. Bipolar neuron- The neuron has the cell body from which arises two neurites called the dendron and the axon respectively. 3. Multipolar neuron- From the cell body arise many dendrites and one long axon. Neurons can also be classified into 1) myelinated when myelin sheath surrounds the axon and 2) non-myelinated neurons when the axon is naked and not covered by the myelin sheath. 1 Structure of a myelinated neuron/nerve cell The neuron can be divided into three parts-cell body or cyton from which arises two types of branches, i.e., many branched processes called dendrons and a long unbranched process called axon. The cyton or cell body is filled with the cytoplasm also called neuroplasm that is limited by the plasma membrane. It contains the nucleus, mitochondria, endoplasmic reticulum, golgi bodies, neurofibrils (filaments that act as a cytoskeleton) and special dense RNA rich granules called Nissl’s granules (help in protein synthesis). The dendrons terminate in slender processes called dendrites. -

1) the Resting Membrane Potential Is: A) 0 Mv, Charge Is Balanced at Rest
1) The resting membrane potential is: a) 0 mV, charge is balanced at rest b) Negative inside because potassium can diffuse out of the cell c) Negative inside because Cl- concentrations are higher inside the cell d) Negative outside because sodium can diffuse into the cell 2) Which of the following is a true statement about cells in the resting state : a) Chloride moves into the cells because the concentration of chloride is higher inside the cell than outside the cell b) Large anions (A-) moves out of the cell because it has a higher concentration inside the cell than outside the cell c) Sodium moves into the cell because it has a higher concentration outside the cell than inside the cell d) Voltage gated sodium channels are closed 3) The action potential threshold stimulus causes: a) chloride to enter the cell b) Voltage gated sodium and potassium channels to open c) Voltage gated sodium channels to inactivate d) Release of Ca++ from the axon 4) Cardiac muscle: a) Is striated b) Has no electrical connection between cells c) Is not striated d) Has cells that are long and unbranched 5) This causes vesicles to migrate and release of neurotransmitter in the chemical synapse: a) Increased dopamine b) Monoamine oxidase c) Increased calcium d) Increased potassium 6) Prozac increases the amount of serotonin present in the synapse by: a) Acting as a selective serotonin reuptake inhibitor b) Increasing tryptophan levels c) Blocking the enzymatic degradation of serotonin d) Increasing serotonin synthesis 7) When the sarcomere shortens: a) Calcium levels decrease b) Myosin pulls on the actin filament c) ATP must not be present d) Potassium is bound to troponin 8) This negatively charged ion is found at high concentrations (i.e., 120 mM) outside the cell: a) Sodium b) A- c) Chloride d) Bicarbonate 9) Excitable cells such as neurons a) Have a resting membrane potential of around +35 mV b) Have a resting membrane potential of around -70 mV c) Have internal potassium concentrations that are the same as potassium concentrations outside the cell. -

Hyperkalemia and Hypocalcemia - Literature Review Modalidades De Parada Cardíaca Induzida: Hipercalemia E Hipocalcemia - Revisão De Literatura
Oliveira MAB,SPECIAL et al. - Modes ARTICLE of induced cardiac arrest: hyperkalemia and Rev Bras Cir Cardiovasc 2014;29(3):432-6 hypocalcemia - Literature review Modes of induced cardiac arrest: hyperkalemia and hypocalcemia - Literature review Modalidades de parada cardíaca induzida: hipercalemia e hipocalcemia - revisão de literatura Marcos Aurélio Barboza de Oliveira1, MD, MsC; Antônio Carlos Brandi2, MD; Carlos Alberto dos Santos2, MD; Paulo Henrique Husseini Botelho2, MD; José Luis Lasso Cortez3, MD; Domingo Marcolino Braile4, MsC, PhD DOI: 10.5935/1678-9741.20140074 RBCCV 44205-1572 Abstract Resumo The entry of sodium and calcium play a key effect on myocyte A entrada de sódio e cálcio desempenham efeito chave no miócito subjected to cardiac arrest by hyperkalemia. They cause cell submetido à parada cardíaca por hiperpotassemia. Eles provocam swelling, acidosis, consumption of adenosine triphosphate and edema celular, acidose, consumo de trifosfato de adenosina e desen- trigger programmed cell death. Cardiac arrest caused by hypo- cadeiam processo de morte celular programada. A parada cardíaca calcemia maintains intracellular adenosine triphosphate levels, provocada por hipocalcemia mantém os níveis intracelulares de improves diastolic performance and reduces oxygen consumption, trifosfato de adenosina, melhora o rendimento diastólico e reduz o which can be translated into better protection to myocyte injury consumo de oxigênio, o que pode ser traduzido em melhor proteção induced by cardiac arrest. do miócito às lesões provocadas pela parada cardíaca induzida. Descriptors: Heart Arrest, Induced. Myocardial Ischemia. Descritores: Parada Cardíaca Induzida. Isquemia Miocárdi- Hyperkalemia. Hypocalcemia. ca. Hiperpotassemia. Hipocalcemia. INTRODUCTION to approach intracardiac diseases[3] by using substances that trigger controlled cardiac arrest, commonly known as cardio- The first cardiac surgeries were performed on a beating plegic agents [4]. -
Ion Channels of the Nervous System Edoxie E
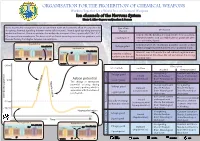
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS Working Together for a World Free of Chemical Weapons Ion channels of the Nervous System Edoxie E. Allier-Gagneur and Jonathan E. Forman Nerve agents and neurotoxins (such as botulinum toxin and saxitoxin) affect life processes by Type of ion Mechanism disrupting chemical signalling between nerve cells (neurons). Neurological signalling processes channel involve ion channels. Those are proteins that enable the transport of ions ( specifically K+, Na+, Ca2+, Activated by the binding of a ligand. In the nervous system, Cl-) across cellular membranes. The direction of ion flow is driven by concentration gradients, with Ligand gated neurotransmitters such as acetylcholine or glutamate often the ions flowing from higher to lower concentration. serve as binding ligands. Depolarization Activated when the membrane potential exceeds or falls Extracellular fluid Voltage-gated + K+ channel K+ Na Na+ channel Repolarization behind a triggering threshold (see action potential chart). Extracellular fluid + + + K channel + Na Na+ channel Allows K ions to flow into the cell while at negative mem- K Inwardly-rectifying/ brane potential. This allows the cell to maintain the resting tandem pore domain + + potential state. Cytoplasm K Na + + Cytoplasm K+ Na - +30 mV Effect when Ion channels Ion Flow Blocked Overstimulated + No signaling. Constant excitation. Voltage-gated Inward Muscle: Paralysis Muscle: Contractions (depolarisation) 0 mV Action potential Ligand-gated Brain: Neurological Brain: Neurological Repolarisation The change in membrane Sodium Na shut-down shut-down potential occuring during No new signal sent. No signaling. Voltage-gated neuronal signaling, which is Outward Muscle: Paralysis Muscle: Paralysis Depolarisation associated with the action of + Brain: Neurological Brain: Neurological Ligand-gated (repolarisation) ion channels.