The Cells That Make Us Who We Are How Neurons Communicate With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
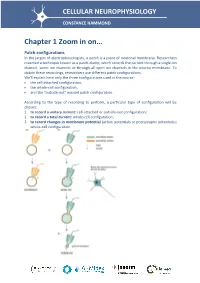
Chapter 1 Zoom in On… Patch Configurations in the Jargon of Electrophysiologists, a Patch Is a Piece of Neuronal Membrane
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 1 Zoom in on… Patch configurations In the jargon of electrophysiologists, a patch is a piece of neuronal membrane. Researchers invented a technique known as a patch-clamp, which records the current through a single ion channel, some ion channels or through all open ion channels in the neuron membrane. To obtain these recordings, researchers use different patch configurations. We'll explain here only the three configurations used in the course: the cell-attached configuration, the whole-cell configuration, and the "outside-out" excised patch configuration. According to the type of recording to perform, a particular type of configuration will be chosen: 1. to record a unitary current: cell-attached or outside-out configuration; 2. to record a total current: whole-cell configuration; 3. to record changes in membrane potential (action potentials or postsynaptic potentials): whole-cell configuration. The cell-attached configuration (attached to the pipette - Figure a) First, the pipette is filled with an extracellular fluid. Positive pressure is applied in the electrode by means of a syringe connected to the electrode, so that the intrapipette fluid tends to leave the pipette. The electrode is then brought near to the soma of a neuron. When the electrode touches the membrane, the positive pressure is withdrawn to draw the membrane toward the mouth of the pipette. We wait without moving the pipette; a seal is made between the walls of the pipette and the soma membrane. This seal strength can be measured by applying a low level of amplitude current in the pipette. Since V = RI, one can measure the resistance of the seal. -

Polyribosomes Associated with Synaptic Specializations on Axon Initial Segments: Localization of Protein-Synthetic Machinery at Inhibitory Synapses
The Journal of Neuroscience October 1986, 6(10): 3079-3085 Polyribosomes Associated with Synaptic Specializations on Axon Initial Segments: Localization of Protein-Synthetic Machinery at Inhibitory Synapses Oswald Steward* and Charles E. Ribak-f *Departments of Neurosurgery and Physiology, University of Virginia School of Medicine, Charlottesville, Virginia 22908, and TDepartment of Anatomy and Neurobiology, University of California at Irvine, Irvine, California 92717 Previous studies have revealed a selective association between report that showed some axonal ribosomes grouped at points polyribosomes and axospinous synapses in a variety of brain of branching and beneath synaptic contacts (Jones and Powell, regions. The present study evaluates whether polyribosomes are 1969). Therefore, they were usually considered, only in passing, also associated with the symmetrical and presumably inhibitory as exceptions to the rule. Thus, it has come to be accepted as a synaptic connections found on the initial segment of axons of central tenet that essentially all of the protein constituents of some neurons in the CNS. The initial segments of pyramidal the neuron, including those required for synaptic specializations, neurons in the sensorimotor cortex of the monkey and of granule were synthesized in the cell body, and somehow selectively cells in the hippocampus of the rat were examined. The initial transported to the sites at which they would be assembled (for segments of these cell types are contacted by GABAergic ter- a review, see Grafstein and Forman, 1980). minals that form symmetrical synaptic connections. In the pres- Over the past few years, a series of observations has given ent study, these initial segments were found to contain polyri- rise to an hypothesis that challenges the prevailing view that bosomes that tended to be selectively localized beneath the virtually all neuronal proteins are produced in the cell body and synaptic specializations. -

Nernst Potentials and Membrane Potential Changes
UNDERSTANDING MEMBRANE POTENTIAL CHANGES IN TERMS OF NERNST POTENTIALS: For seeing how a change in conductance to ions affects the membrane potential, follow these steps: 1. Make a graph with membrane potential on the vertical axis (-100 to +55) and time on the horizontal axis. 2. Draw dashed lines indicating the standard Nernst potential (equilibrium potential) for each ion: Na+ = +55 mV, K+ = -90mV, Cl- = -65 mV. 3. Draw lines below the horizontal axis showing the increased conductance to individual ions. 4. Start plotting the membrane potential on the left. Most graphs will start at resting potential (-70 mV) 5. When current injection (Stim) is present, move the membrane potential upward to Firing threshold. 6. For the time during which membrane conductance to a particular ion increases, move the membrane potential toward the Nernst potential for that ion. 7. During the time when conductance to a particular ion decreases, move the membrane potential away from the Nernst potential of that ion, toward a position which averages the conductances of the other ions. 8. When conductances return to their original value, membrane potential will go to its starting value. +55mV ACTION POTENTIAL SYNAPTIC POTENTIALS 0mV Membrane potential Firing threshold Firing threshold -65mV -90mV Time Time Na+ K+ Conductances Stim CHANGES IN MEMBRANE POTENTIAL ALLOW NEURONS TO COMMUNICATE The membrane potential of a neuron can be measured with an intracellular electrode. This 1 provides a measurement of the voltage difference between the inside of the cell and the outside. When there is no external input, the membrane potential will usually remain at a value called the resting potential. -

Effect of Hyperkalemia on Membrane Potential: Depolarization
❖ CASE 3 A 6-year-old boy is brought to the family physician after his parents noticed that he had difficulty moving his arms and legs after a soccer game. About 10 minutes after leaving the field, the boy became so weak that he could not stand for about 30 minutes. Questioning revealed that he had complained of weakness after eating bananas, had frequent muscle spasms, and occasionally had myotonia, which was expressed as difficulty in releasing his grip or diffi- culty opening his eyes after squinting into the sun. After a thorough physical examination, the boy was diagnosed with hyperkalemic periodic paralysis. The family was advised to feed the boy carbohydrate-rich, low-potassium foods, give him glucose-containing drinks during attacks, and have him avoid strenuous exercise and fasting. ◆ What is the effect of hyperkalemia on cell membrane potential? ◆ What is responsible for the repolarizing phase of an action potential? ◆ What is the effect of prolonged depolarization on the skeletal muscle Na+ channel? 32 CASE FILES: PHYSIOLOGY ANSWERS TO CASE 3: ACTION POTENTIAL Summary: A 6-year-old boy who experiences profound weakness after exer- cise is diagnosed with hyperkalemic periodic paralysis. ◆ Effect of hyperkalemia on membrane potential: Depolarization. ◆ Repolarization mechanisms: Activation of voltage-gated K+ conductance and inactivation of Na+ conductance. ◆ Effect of prolonged depolarization: Inactivation of Na+ channels. CLINICAL CORRELATION Hyperkalemic periodic paralysis (HyperPP) is a dominant inherited trait caused by a mutation in the α subunit of the skeletal muscle Na+ channel. It occurs in approximately 1 in 100,000 people and is more common and more severe in males. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -

11 Introduction to the Nervous System and Nervous Tissue
11 Introduction to the Nervous System and Nervous Tissue ou can’t turn on the television or radio, much less go online, without seeing some- 11.1 Overview of the Nervous thing to remind you of the nervous system. From advertisements for medications System 381 Yto treat depression and other psychiatric conditions to stories about celebrities and 11.2 Nervous Tissue 384 their battles with illegal drugs, information about the nervous system is everywhere in 11.3 Electrophysiology our popular culture. And there is good reason for this—the nervous system controls our of Neurons 393 perception and experience of the world. In addition, it directs voluntary movement, and 11.4 Neuronal Synapses 406 is the seat of our consciousness, personality, and learning and memory. Along with the 11.5 Neurotransmitters 413 endocrine system, the nervous system regulates many aspects of homeostasis, including 11.6 Functional Groups respiratory rate, blood pressure, body temperature, the sleep/wake cycle, and blood pH. of Neurons 417 In this chapter we introduce the multitasking nervous system and its basic functions and divisions. We then examine the structure and physiology of the main tissue of the nervous system: nervous tissue. As you read, notice that many of the same principles you discovered in the muscle tissue chapter (see Chapter 10) apply here as well. MODULE 11.1 Overview of the Nervous System Learning Outcomes 1. Describe the major functions of the nervous system. 2. Describe the structures and basic functions of each organ of the central and peripheral nervous systems. 3. Explain the major differences between the two functional divisions of the peripheral nervous system. -
4-Nervous-System-Structure-PPT.Pdf
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation and coordination that produce coherency and result in harmonious function. Integration Cellular integration = processes within cells Whole-animal integration = selective combination and processing of sensory, endocrine, and central nervous system (CNS) information in ways that promote harmonious functioning of the whole organism within its environment. ◦ This includes its all its cells, tissues, and organs Integration Nerve cells are specialized for control and coordination. Integration ensures that an animal’s responses are smooth and coordinated rather than clashing or disjointed. Excitable Cells Neurons are a type of excitable cell ◦ Specially adapted to generate an electrical signal Can rapidly alter their membrane potential in response to an incoming signal. Vary in structure and function but use the same basic mechanism to send signals. Neuron Function – Main Points Specialized for processing and conveying information. Information is coded into changes in electrical potential across cell membranes. Neurons use action potentials to transmit these signals across long distances. Neurons allow animals to sense and respond to their environment. Benefits of Neurons Plants (no neurons): Action potentials travel @ 1-3 cm/sec Animals (neurons): Action potentials travel @ 100m/sec or 10,000cm/sec CNS to Muscles Signal Reception Dendrites & Cell Body Signal Integration Axon Hillock Signal Conduction Axon Signal Transmission Axon Terminals Signal Reception Dendrites sense and convert incoming signals into electrical signals by changing membrane potential. Cell Body = routine metabolic functions Signal Integration Incoming signals are conducted to the axon hillock If signal is sufficiently large an electrical signal, or action potential, is initiated. -
The Action Potential
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/6316219 The action potential ARTICLE in PRACTICAL NEUROLOGY · JULY 2007 Source: PubMed CITATIONS READS 16 64 2 AUTHORS, INCLUDING: Mark W Barnett The University of Edinburgh 21 PUBLICATIONS 661 CITATIONS SEE PROFILE Available from: Mark W Barnett Retrieved on: 24 October 2015 192 Practical Neurology HOW TO UNDERSTAND IT Pract Neurol 2007; 7: 192–197 The action potential Mark W Barnett, Philip M Larkman t is over 60 years since Hodgkin and called ion channels that form the permeation Huxley1 made the first direct recording of pathways across the neuronal membrane. the electrical changes across the neuro- Although the first electrophysiological nal membrane that mediate the action recordings from individual ion channels were I 2 potential. Using an electrode placed inside a not made until the mid 1970s, Hodgkin and squid giant axon they were able to measure a Huxley predicted many of the properties now transmembrane potential of around 260 mV known to be key components of their inside relative to outside, under resting function: ion selectivity, the electrical basis conditions (this is called the resting mem- of voltage-sensitivity and, importantly, a brane potential). The action potential is a mechanism for quickly closing down the transient (,1 millisecond) reversal in the permeability pathways to ensure that the polarity of this transmembrane potential action potential only moves along the axon in which then moves from its point -

Membrane Potentials As Signals
Membrane Potentials as Signals The membrane potential allows a cell to function as a battery, providing electrical power to activities within the cell and between cells. KEY POINTS A membrane potential is the difference in electrical potential between the interior and the exterior of a biological cell. In electrically excitable cells, changes in membrane potential are used for transmitting signals within the cell. The opening and closing of ion channels can induce changes from the resting potential. Depolarization is when the interior voltage becomes more positive and hyperpolarization when it becomes more negative. TERMS Graded potentials Graded potentials vary in size and arise from the summation of the individual actions of ligand- gated ion channel proteins, and decrease over time and space. Depolarization Depolarization is a change in a cell's membrane potential, making it more positive, or less negative, and may result in generation of an action potential. Action potential A short term change in the electrical potential that travels along a cell such as a nerve or muscle fiber. EXAMPLE In neurons, a sufficiently large depolarization can evoke an action potential in which the membrane potential changes rapidly. Give us feedback on this content: Membrane potential (also transmembrane potential or membrane voltage) is the difference in electrical potential between the interior and the exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions. -

1 Probing the Membrane Potential of Living Cells by Dielectric
Probing the membrane potential of living cells by dielectric spectroscopy Corina Bot and Camelia Prodan Dept. of Physics, New Jersey Inst. of Techn, Newark, NJ Abstract. In this paper we demonstrate a quantitative way to measure the membrane potential of live cells by dielectric spectroscopy. We also show that the values of the membrane potential obtained using our technique are in good agreement with those obtained using traditional methods-voltage sensitive dyes. The membrane potential is determined by fitting the experimental dielectric dispersion curves with the dispersion curves obtain from a theoretical model. Variations in the membrane potential were induced by modifying the concentration of potassium chloride in the solution of the cell suspension in the presence of valinomycin. For exemplification of the method, E. coli were chosen for our experiments. Introduction The resting membrane potential is one of the most important living cell parameters. By monitoring a cell’s membrane potential, one can get important information about its state. Changes in the membrane potential have been linked to different diseases, including Parkinson’s, epilepsy and Bartter’s syndrome [1]. A decrease in the plasma membrane potential has been seen in tumor cells with increased expression of multi-drug resistance (MDR) protein, also known as P-glycoprotein [2]. It was also shown that the membrane potential plays a critical role in the antibiotic uptake by cells and in bactericidal action [3]. Thus measuring or monitoring the membrane potential is of great importance. The existing “gold” or standard methods to measure or monitor the membrane potential are patch clamping and voltage sensitive dyes. -
Nervous System
Nervous System Nervous Tissue l Master integrating and control system of body l Composed of 2 main parts l Central nervous system consisting of brain and spinal cord l Peripheral nervous system consisting of nerves, ganglia and receptors Basic terminology l Neuron is term for “nerve cell” l Supporting cells are called glial cells: they protect the delicate neurons (see pg 254 ) – Central Nervous System (CNS) l Astrocytes, oligodendrocytes, microglia and ependymal cells – Peripheral Nervous System (PNS) l Schwann cells and satellite cells Neuroglia of CNS l Astrocytes: blood brain barrier, maintain chemical environment, metabolize neurotransmitters, regulate K+ levels, provide structural support l Oligodendrocytes: myelin sheath l Microglia: phagocytic cells l Ependymal cells – form and circulate CSF Neuroglia of PNS l Schwann cells – produce myelin sheath in CNS l Satellite cells – structural support for neuron cell bodies in PNS, regulate exchange material between cell bodies and interstitial fluid. Neuron Anatomy 1. Cell body has nucleus – Usually found in the CNS in clusters called nuclei – Sometimes found outside CNS in areas called ganglia – has neurofibrils (bundles of intermediate filaments;part of cytoskeleton) – Nissl bodies (clusters of endoplasmic reticulum) – Lipofuschin yellowish brown cytoplasmic granules, a product of lysosomes Cont… 2. Dendrites are multiple short, branching neuronal processes that RECEIVE electrical signals 3. Axons is a long, usually single neuron process that GENERATE electrical signals 4. Axon hillock is where the axon begins on the neuron cell body 5. Initial segment is first part of axon 6. Trigger zone at junction of axon hillock and initial segment, impulses arise here 7. Axoplasm – cytoplasm of axon 8. -

The Neuronal Endomembrane System III
0270.6474/85/0512-3135$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5, No. 12. pp. 3135-3144 Pnnted in U.S.A. December 1985 The Neuronal Endomembrane System III. The Origins of Axoplasmic Reticulum Discrete Axonal Cisternae the Axon Hillock’ JAMES D. LINDSEY2 AND MARK H. ELLISMAN Department of Neurosciences, University of California, San Diego, School of Medicine, La Jolla, California 92093 Abstract The axoplasmic reticulum (AR) and the discrete element vesicles were usually found in close association with the (e.g., vesicles, vesiculotubular bodies, multivesicular bodies, trans face of the Golgi apparatus. These results indirectly etc.) constitute the endomembrane system of the axon. It is support the hypothesis that vectors of fast axonal transport, reported here that the AR of bullfrog sciatic nerve readily fills namely the discrete elements, form directly at the trans face with osmium deposits during osmium impregnation. In con- of the Golgi apparatus. From here they move toward and trast, the discrete elements and mitochondria are highly subsequently down the axon without any membrane fission- resistant to impregnation. Hence this preparation is well fusion events with either RER or AR. AR, although it forms suited to address the nature of possible interactions between continuities with RER, retains a distinctly different chemical AR and rough endoplasmic reticulum (RER) in the axon composition from RER as evidenced by its much higher hillock. It is also ideal to study the origin of the axonal discrete affinity for osmium. Thus, it should be considered as an elements within the cell body as well as their interaction with endomembrane component separate from, although inti- other somal endomembrane system components.