A Strongly Inwardly Rectifying K+ Channel That Is Sensitive to ATP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
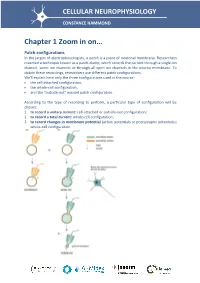
Chapter 1 Zoom in On… Patch Configurations in the Jargon of Electrophysiologists, a Patch Is a Piece of Neuronal Membrane
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 1 Zoom in on… Patch configurations In the jargon of electrophysiologists, a patch is a piece of neuronal membrane. Researchers invented a technique known as a patch-clamp, which records the current through a single ion channel, some ion channels or through all open ion channels in the neuron membrane. To obtain these recordings, researchers use different patch configurations. We'll explain here only the three configurations used in the course: the cell-attached configuration, the whole-cell configuration, and the "outside-out" excised patch configuration. According to the type of recording to perform, a particular type of configuration will be chosen: 1. to record a unitary current: cell-attached or outside-out configuration; 2. to record a total current: whole-cell configuration; 3. to record changes in membrane potential (action potentials or postsynaptic potentials): whole-cell configuration. The cell-attached configuration (attached to the pipette - Figure a) First, the pipette is filled with an extracellular fluid. Positive pressure is applied in the electrode by means of a syringe connected to the electrode, so that the intrapipette fluid tends to leave the pipette. The electrode is then brought near to the soma of a neuron. When the electrode touches the membrane, the positive pressure is withdrawn to draw the membrane toward the mouth of the pipette. We wait without moving the pipette; a seal is made between the walls of the pipette and the soma membrane. This seal strength can be measured by applying a low level of amplitude current in the pipette. Since V = RI, one can measure the resistance of the seal. -

Chemical Synthesis, Proper Folding, Nav Channel Selectivity Profile And
toxins Article Chemical Synthesis, Proper Folding, Nav Channel Selectivity Profile and Analgesic Properties of the Spider Peptide Phlotoxin 1 1, 2,3, 4,5, 1 Sébastien Nicolas y, Claude Zoukimian y, Frank Bosmans y,Jérôme Montnach , Sylvie Diochot 6, Eva Cuypers 5, Stephan De Waard 1,Rémy Béroud 2, Dietrich Mebs 7 , David Craik 8, Didier Boturyn 3 , Michel Lazdunski 6, Jan Tytgat 5 and Michel De Waard 1,2,* 1 Institut du Thorax, Inserm UMR 1087/CNRS UMR 6291, LabEx “Ion Channels, Science & Therapeutics”, F-44007 Nantes, France; [email protected] (S.N.); [email protected] (J.M.); [email protected] (S.D.W.) 2 Smartox Biotechnology, 6 rue des Platanes, F-38120 Saint-Egrève, France; [email protected] (C.Z.); [email protected] (R.B.) 3 Department of Molecular Chemistry, Univ. Grenoble Alpes, CNRS, 570 rue de la chimie, CS 40700, 38000 Grenoble, France; [email protected] 4 Faculty of Medicine and Health Sciences, Department of Basic and Applied Medical Sciences, 9000 Gent, Belgium; [email protected] 5 Toxicology and Pharmacology, University of Leuven, Campus Gasthuisberg, P.O. Box 922, Herestraat 49, 3000 Leuven, Belgium; [email protected] (E.C.); [email protected] (J.T.) 6 Université Côte d’Azur, CNRS UMR7275, Institut de Pharmacologie Moléculaire et Cellulaire, 660 route des lucioles, 6560 Valbonne, France; [email protected] (S.D.); [email protected] (M.L.) 7 Institute of Legal Medicine, University of Frankfurt, Kennedyallee 104, 60488 Frankfurt, Germany; [email protected] 8 Institute for Molecular Bioscience, University of Queensland, Brisbane 4072, Australia; [email protected] * Correspondence: [email protected]; Tel.: +33-228-080-076 Contributed equally to this work. -

Nernst Potentials and Membrane Potential Changes
UNDERSTANDING MEMBRANE POTENTIAL CHANGES IN TERMS OF NERNST POTENTIALS: For seeing how a change in conductance to ions affects the membrane potential, follow these steps: 1. Make a graph with membrane potential on the vertical axis (-100 to +55) and time on the horizontal axis. 2. Draw dashed lines indicating the standard Nernst potential (equilibrium potential) for each ion: Na+ = +55 mV, K+ = -90mV, Cl- = -65 mV. 3. Draw lines below the horizontal axis showing the increased conductance to individual ions. 4. Start plotting the membrane potential on the left. Most graphs will start at resting potential (-70 mV) 5. When current injection (Stim) is present, move the membrane potential upward to Firing threshold. 6. For the time during which membrane conductance to a particular ion increases, move the membrane potential toward the Nernst potential for that ion. 7. During the time when conductance to a particular ion decreases, move the membrane potential away from the Nernst potential of that ion, toward a position which averages the conductances of the other ions. 8. When conductances return to their original value, membrane potential will go to its starting value. +55mV ACTION POTENTIAL SYNAPTIC POTENTIALS 0mV Membrane potential Firing threshold Firing threshold -65mV -90mV Time Time Na+ K+ Conductances Stim CHANGES IN MEMBRANE POTENTIAL ALLOW NEURONS TO COMMUNICATE The membrane potential of a neuron can be measured with an intracellular electrode. This 1 provides a measurement of the voltage difference between the inside of the cell and the outside. When there is no external input, the membrane potential will usually remain at a value called the resting potential. -

An Improved Voltage Clamp Circuit Suitable for Accurate Measurement of the Conduction Loss of Power Electronic Devices
sensors Article An Improved Voltage Clamp Circuit Suitable for Accurate Measurement of the Conduction Loss of Power Electronic Devices Qiuping Yu, Zhibin Zhao *, Peng Sun, Bin Zhao and Yumeng Cai State Key Laboratory of Alternate Electrical Power System with Renewable Energy Sources, North China Electric Power University, Beijing 102206, China; [email protected] (Q.Y.); [email protected] (P.S.); [email protected] (B.Z.); [email protected] (Y.C.) * Correspondence: [email protected] Abstract: Power electronic devices are essential components of high-capacity industrial converters. Accurate assessment of their power loss, including switching loss and conduction loss, is essential to improving electrothermal stability. To accurately calculate the conduction loss, a drain–source voltage clamp circuit is required to measure the on-state voltage. In this paper, the conventional drain–source voltage clamp circuit based on a transistor is comprehensively investigated by theoretical analysis, simulations, and experiments. It is demonstrated that the anti-parallel diodes and the gate-shunt capacitance of the conventional drain–source voltage clamp circuit have adverse impacts on the accuracy and security of the conduction loss measurement. Based on the above analysis, an improved drain–source voltage clamp circuit, derived from the conventional drain–source voltage clamp circuit, is proposed to solve the above problems. The operational advantages, physical structure, and design guidelines of the improved circuit are fully presented. In addition, to evaluate the influence of component parameters on circuit performance, this article comprehensively extracts three electrical Citation: Yu, Q.; Zhao, Z.; Sun, P.; quantities as judgment indicators. Based on the working mechanism of the improved circuit and Zhao, B.; Cai, Y. -

Lecture 1: an Introduction to Plasticity and Cellular Electrophysiology
Lecture 1: An Introduction To Plasticity and Cellular Electrophysiology MCP 2003 Charge separation across a ATPase + semipermeable Ca++ 3Na ATPase membrane is the 2K+ (mM) basis of excitability. K+ = 140 + Na = 7 ++ + Ca Cl- = 7 3Na (mM) Ca++ = 1 x 10-4 co-transporter K+ =3 Na+ = 140 membrane pores Cl- = 140 with variable Ca++ = 1.5 permeability - + R - + m Cm gK+ gCl- gNa++ Convention: Current direction is defined by the direction of increasing positive charge. Na++ flux into a cell is an inward current. K+ flux out of a cell is an outward current. Cl- flux into a cell is an outward current. A depolarizing current is a net influx of + ions or a net efflux of negative ions. A hyperpolarizing current is a net efflux of + ions or a net influx of negative ions. I Outward rectification: when a membrane allows outward current (net + charge out) V to flow more easily than and inward current. Outward rectification I Inward rectification: when a membrane allows inward current to flow more easily V than an outward current. Inward rectification Methods of Measuring Function In Excitable Membranes Field potentials: measure current sources _ low pass filter and sinks from populations of neurons + across the electrode resistance. _ 0 Brain mV-V - t (sec) + Microelectrode extracellular recording: measures action potentials from a small _ number of neurons. 0 mV pulse amplitude window - Intracellular recording: can measure voltage, t (msec) band pass filter 1KHz-10KHz +40 0 Voltage clamp recording: resting membrane Can pass current to compensate -60 potential for voltage change. In this way voltage is held ~ constant and current applied to _ compensate is a measure of the current flowing. -

Effect of Hyperkalemia on Membrane Potential: Depolarization
❖ CASE 3 A 6-year-old boy is brought to the family physician after his parents noticed that he had difficulty moving his arms and legs after a soccer game. About 10 minutes after leaving the field, the boy became so weak that he could not stand for about 30 minutes. Questioning revealed that he had complained of weakness after eating bananas, had frequent muscle spasms, and occasionally had myotonia, which was expressed as difficulty in releasing his grip or diffi- culty opening his eyes after squinting into the sun. After a thorough physical examination, the boy was diagnosed with hyperkalemic periodic paralysis. The family was advised to feed the boy carbohydrate-rich, low-potassium foods, give him glucose-containing drinks during attacks, and have him avoid strenuous exercise and fasting. ◆ What is the effect of hyperkalemia on cell membrane potential? ◆ What is responsible for the repolarizing phase of an action potential? ◆ What is the effect of prolonged depolarization on the skeletal muscle Na+ channel? 32 CASE FILES: PHYSIOLOGY ANSWERS TO CASE 3: ACTION POTENTIAL Summary: A 6-year-old boy who experiences profound weakness after exer- cise is diagnosed with hyperkalemic periodic paralysis. ◆ Effect of hyperkalemia on membrane potential: Depolarization. ◆ Repolarization mechanisms: Activation of voltage-gated K+ conductance and inactivation of Na+ conductance. ◆ Effect of prolonged depolarization: Inactivation of Na+ channels. CLINICAL CORRELATION Hyperkalemic periodic paralysis (HyperPP) is a dominant inherited trait caused by a mutation in the α subunit of the skeletal muscle Na+ channel. It occurs in approximately 1 in 100,000 people and is more common and more severe in males. -

Cholesterol Modulates the Recruitment of Kv1.5 Channels from Rab11-Associated Recycling Endosome in Native Atrial Myocytes
Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes Elise Balsea,b, Saïd El-Haoua,b, Gilles Dillaniana,b, Aure´ lien Dauphinc, Jodene Eldstromd, David Fedidad, Alain Coulombea,b, and Ste´ phane N. Hatema,b,1 aInstitut National de la Sante´et de la Recherche Me´dicale, Unite´Mixte de Recherche Scientifique-956, 75013 Paris, France; bUniversite´Pierre et Marie Curie, Paris-6, Unite´Mixte de Recherche Scientifique-956, 75013 Paris, France; cPlate-forme imagerie cellulaire IFR14, 75013 Paris, France; and dDepartment of Anesthesiology, Pharmacology and Therapeutics, University of British Columbia, Vancouver, BC, Canada V6T 1Z3 Edited by Lily Y. Jan, University of California, San Francisco, CA, and approved June 19, 2009 (received for review March 17, 2009) Cholesterol is an important determinant of cardiac electrical proper- important role in the regulation of expression of KCNQ1/KCNE1, ties. However, underlying mechanisms are still poorly understood. pacemaker HCN channels and Kv1.5 channels. This process in- Here, we examine the hypothesis that cholesterol modulates the volves several Rab-GTPases (8–10). Rab-GTPases regulate the turnover of voltage-gated potassium channels based on previous trafficking of vesicles between plasma membrane and intracellular observations showing that depletion of membrane cholesterol in- compartments by regulating sorting, tethering and docking of creases the atrial repolarizing current IKur. Whole-cell currents and trafficking vesicles. Rab4, associated with the early endosome (EE), single-channel activity were recorded in rat adult atrial myocytes mediates the fast recycling process while Rab11, linked to the (AAM) or after transduction with hKv1.5-EGFP. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -

A Randomized, Double-Blind, Placebo Controlled, Cross-Over Trial of Quinidine in Genetic Epilepsy Due to KCNT1 Mutations
A trial of quinidine in genetic epilepsy A randomized, double-blind, placebo controlled, cross-over trial of quinidine in genetic epilepsy due to KCNT1 mutations Principal Investigator – Dr Saul Mullen Associate Investigators – Prof Ingrid Scheffer, Prof Samuel Berkovic, Dr Patrick Carney, Version 4 19th November, 2014 Page 1 of 14 Version 4, November 2014 A trial of quinidine in genetic epilepsy Introduction Epilepsy is defined by repeated, unprovoked seizures and is a major global health problem with a lifetime incidence of over 3% 1. Epilepsy is not, however, a uniform condition but rather a collection of syndromes with widely variable course, severity and underlying causes. Amongst these causes of epilepsy, inherited factors are prominent. The genetics of most inherited epilepsies is likely complex with multiple genes interacting with environmental factors to produce disease. An increasing number of monogenic epilepsies are however recognised. The majority of genes carrying epilepsy- causing mutations are neuronal ion channel subunits, thus leading to effects on either synaptic transmission or action potential firing. At present, the vast majority of epilepsy therapies take little account of the underlying causes. Anti- epileptic drugs are largely developed and tested in broad cohorts of epilepsy with mixed aetiologies. This has led to drugs each individually usable in most patients but with modest efficacy at best. Tailored drug therapies are starting to emerge for genetic epilepsies. For instance, in tuberous sclerosis complex, genetic abnormalities of the functional cascade associated with Mammalian Target of Rapamycin (mTOR) protein lead to the disease. Treatment with a specific inhibitor of this pathway, everolimus, leads to improved outcomes both in terms of seizures and secondary development of tumours 2. -
The Action Potential
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/6316219 The action potential ARTICLE in PRACTICAL NEUROLOGY · JULY 2007 Source: PubMed CITATIONS READS 16 64 2 AUTHORS, INCLUDING: Mark W Barnett The University of Edinburgh 21 PUBLICATIONS 661 CITATIONS SEE PROFILE Available from: Mark W Barnett Retrieved on: 24 October 2015 192 Practical Neurology HOW TO UNDERSTAND IT Pract Neurol 2007; 7: 192–197 The action potential Mark W Barnett, Philip M Larkman t is over 60 years since Hodgkin and called ion channels that form the permeation Huxley1 made the first direct recording of pathways across the neuronal membrane. the electrical changes across the neuro- Although the first electrophysiological nal membrane that mediate the action recordings from individual ion channels were I 2 potential. Using an electrode placed inside a not made until the mid 1970s, Hodgkin and squid giant axon they were able to measure a Huxley predicted many of the properties now transmembrane potential of around 260 mV known to be key components of their inside relative to outside, under resting function: ion selectivity, the electrical basis conditions (this is called the resting mem- of voltage-sensitivity and, importantly, a brane potential). The action potential is a mechanism for quickly closing down the transient (,1 millisecond) reversal in the permeability pathways to ensure that the polarity of this transmembrane potential action potential only moves along the axon in which then moves from its point -

Recording Techniques
Electrophysiological Recording Techniques Wen-Jun Gao, PH.D. Drexel University College of Medicine Goal of Physiological Recording To detect the communication signals between neurons in real time (μs to hours) • Current clamp – measure membrane potential, PSPs, action potentials, resting membrane potential • Voltage clamp – measure membrane current, PSCs, voltage-ligand activated conductances 1 Current is conserved at a branch point A Typical Electrical Circuit Example of an electrical circuit with various parts. Current always flows in a complete circuit. 2 Resistors and Conductors Summation of Conductance: Conductances in parallel summate together, whether they are resistors or channels. Ohm's Law For electrophysiology, perhaps the most important law of electricity is Ohm's law. The potential difference between two points linked by a current path with a conductance G and a current I is: 3 Representative Voltmeter with Infinite Resistance Instruments used to measure potentials must have a very high input resistance Rin. Capacitors and Their Electrical Fields A charge Q is stored in a capacitor of value C held at a potential DeltaV. Q = C* delta V capacitance 4 Capacitors in Parallel Add Their Values Currents Through Capacitors Membrane Behavior Compared with an Electrical Current A A membrane behaves electrically like a capacitance in parallel with a resistance. B Now, if we apply a pulse of current to the circuit, the current first charges up the Response of an RC parallel capacitance, then changes circuit to a step of current the voltage 5 The voltage V(t) approaches steady state along an exponential time course: The steady-state value Vinf (also called the infinite-time or equilibrium value) does not depend on the capacitance; it is simply determined by the current I and the membrane resistance R: This is just Ohm's law, of course; but when the membrane capacitance is in the circuit, the voltage is not reached immediately. -

Membrane Potentials As Signals
Membrane Potentials as Signals The membrane potential allows a cell to function as a battery, providing electrical power to activities within the cell and between cells. KEY POINTS A membrane potential is the difference in electrical potential between the interior and the exterior of a biological cell. In electrically excitable cells, changes in membrane potential are used for transmitting signals within the cell. The opening and closing of ion channels can induce changes from the resting potential. Depolarization is when the interior voltage becomes more positive and hyperpolarization when it becomes more negative. TERMS Graded potentials Graded potentials vary in size and arise from the summation of the individual actions of ligand- gated ion channel proteins, and decrease over time and space. Depolarization Depolarization is a change in a cell's membrane potential, making it more positive, or less negative, and may result in generation of an action potential. Action potential A short term change in the electrical potential that travels along a cell such as a nerve or muscle fiber. EXAMPLE In neurons, a sufficiently large depolarization can evoke an action potential in which the membrane potential changes rapidly. Give us feedback on this content: Membrane potential (also transmembrane potential or membrane voltage) is the difference in electrical potential between the interior and the exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions.