1 Ions, Channels, and Currents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
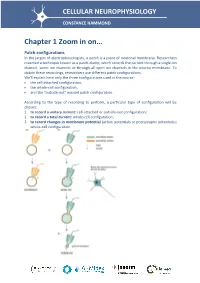
Chapter 1 Zoom in On… Patch Configurations in the Jargon of Electrophysiologists, a Patch Is a Piece of Neuronal Membrane
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 1 Zoom in on… Patch configurations In the jargon of electrophysiologists, a patch is a piece of neuronal membrane. Researchers invented a technique known as a patch-clamp, which records the current through a single ion channel, some ion channels or through all open ion channels in the neuron membrane. To obtain these recordings, researchers use different patch configurations. We'll explain here only the three configurations used in the course: the cell-attached configuration, the whole-cell configuration, and the "outside-out" excised patch configuration. According to the type of recording to perform, a particular type of configuration will be chosen: 1. to record a unitary current: cell-attached or outside-out configuration; 2. to record a total current: whole-cell configuration; 3. to record changes in membrane potential (action potentials or postsynaptic potentials): whole-cell configuration. The cell-attached configuration (attached to the pipette - Figure a) First, the pipette is filled with an extracellular fluid. Positive pressure is applied in the electrode by means of a syringe connected to the electrode, so that the intrapipette fluid tends to leave the pipette. The electrode is then brought near to the soma of a neuron. When the electrode touches the membrane, the positive pressure is withdrawn to draw the membrane toward the mouth of the pipette. We wait without moving the pipette; a seal is made between the walls of the pipette and the soma membrane. This seal strength can be measured by applying a low level of amplitude current in the pipette. Since V = RI, one can measure the resistance of the seal. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -

Current, TTX-Resistant Na؉ Current, and Ca2؉ Current in the Action Potentials of Nociceptive Sensory Neurons
The Journal of Neuroscience, December 1, 2002, 22(23):10277–10290 Roles of Tetrodotoxin (TTX)-Sensitive Na؉ Current, TTX-Resistant Na؉ Current, and Ca2؉ Current in the Action Potentials of Nociceptive Sensory Neurons Nathaniel T. Blair and Bruce P. Bean Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 20114 Nociceptive sensory neurons are unusual in expressing carry the largest inward charge during the upstroke of the voltage-gated inward currents carried by sodium channels re- nociceptor action potential (ϳ58%), with TTX-sensitive sodium sistant to block by tetrodotoxin (TTX) as well as currents carried channels also contributing significantly (ϳ40%), especially near by conventional TTX-sensitive sodium channels and voltage- threshold, and high voltage-activated calcium currents much dependent calcium channels. To examine how currents carried less (ϳ2%). Action potentials had a prominent shoulder during by each of these helps to shape the action potential in small- the falling phase, characteristic of nociceptive neurons. TTX- diameter dorsal root ganglion cell bodies, we voltage clamped resistant sodium channels did not inactivate completely during cells by using the action potential recorded from each cell as the action potential and carried the majority (58%) of inward the command voltage. Using intracellular solutions of physio- current flowing during the shoulder, with high voltage-activated logical ionic composition, we isolated individual components of calcium current also contributing significantly (39%). -

Nernst Potentials and Membrane Potential Changes
UNDERSTANDING MEMBRANE POTENTIAL CHANGES IN TERMS OF NERNST POTENTIALS: For seeing how a change in conductance to ions affects the membrane potential, follow these steps: 1. Make a graph with membrane potential on the vertical axis (-100 to +55) and time on the horizontal axis. 2. Draw dashed lines indicating the standard Nernst potential (equilibrium potential) for each ion: Na+ = +55 mV, K+ = -90mV, Cl- = -65 mV. 3. Draw lines below the horizontal axis showing the increased conductance to individual ions. 4. Start plotting the membrane potential on the left. Most graphs will start at resting potential (-70 mV) 5. When current injection (Stim) is present, move the membrane potential upward to Firing threshold. 6. For the time during which membrane conductance to a particular ion increases, move the membrane potential toward the Nernst potential for that ion. 7. During the time when conductance to a particular ion decreases, move the membrane potential away from the Nernst potential of that ion, toward a position which averages the conductances of the other ions. 8. When conductances return to their original value, membrane potential will go to its starting value. +55mV ACTION POTENTIAL SYNAPTIC POTENTIALS 0mV Membrane potential Firing threshold Firing threshold -65mV -90mV Time Time Na+ K+ Conductances Stim CHANGES IN MEMBRANE POTENTIAL ALLOW NEURONS TO COMMUNICATE The membrane potential of a neuron can be measured with an intracellular electrode. This 1 provides a measurement of the voltage difference between the inside of the cell and the outside. When there is no external input, the membrane potential will usually remain at a value called the resting potential. -

Effect of Hyperkalemia on Membrane Potential: Depolarization
❖ CASE 3 A 6-year-old boy is brought to the family physician after his parents noticed that he had difficulty moving his arms and legs after a soccer game. About 10 minutes after leaving the field, the boy became so weak that he could not stand for about 30 minutes. Questioning revealed that he had complained of weakness after eating bananas, had frequent muscle spasms, and occasionally had myotonia, which was expressed as difficulty in releasing his grip or diffi- culty opening his eyes after squinting into the sun. After a thorough physical examination, the boy was diagnosed with hyperkalemic periodic paralysis. The family was advised to feed the boy carbohydrate-rich, low-potassium foods, give him glucose-containing drinks during attacks, and have him avoid strenuous exercise and fasting. ◆ What is the effect of hyperkalemia on cell membrane potential? ◆ What is responsible for the repolarizing phase of an action potential? ◆ What is the effect of prolonged depolarization on the skeletal muscle Na+ channel? 32 CASE FILES: PHYSIOLOGY ANSWERS TO CASE 3: ACTION POTENTIAL Summary: A 6-year-old boy who experiences profound weakness after exer- cise is diagnosed with hyperkalemic periodic paralysis. ◆ Effect of hyperkalemia on membrane potential: Depolarization. ◆ Repolarization mechanisms: Activation of voltage-gated K+ conductance and inactivation of Na+ conductance. ◆ Effect of prolonged depolarization: Inactivation of Na+ channels. CLINICAL CORRELATION Hyperkalemic periodic paralysis (HyperPP) is a dominant inherited trait caused by a mutation in the α subunit of the skeletal muscle Na+ channel. It occurs in approximately 1 in 100,000 people and is more common and more severe in males. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -

Conditional Knockout of Nav1.6 in Adult Mice Ameliorates Neuropathic Pain
www.nature.com/scientificreports OPEN Conditional knockout of NaV1.6 in adult mice ameliorates neuropathic pain Received: 22 November 2017 Lubin Chen 1,2,3, Jianying Huang1,2,3, Peng Zhao1,2,3, Anna-Karin Persson1,2,3, Accepted: 19 February 2018 Fadia B. Dib-Hajj1,2,3, Xiaoyang Cheng1,2,3, Andrew Tan1,2,3, Stephen G. Waxman1,2,3 & Published: xx xx xxxx Sulayman D. Dib-Hajj 1,2,3 Voltage-gated sodium channels NaV1.7, NaV1.8 and NaV1.9 have been the focus for pain studies because their mutations are associated with human pain disorders, but the role of NaV1.6 in pain is less understood. In this study, we selectively knocked out NaV1.6 in dorsal root ganglion (DRG) neurons, using NaV1.8-Cre directed or adeno-associated virus (AAV)-Cre mediated approaches, and examined the specifc contribution of NaV1.6 to the tetrodotoxin-sensitive (TTX-S) current in these neurons and its role in neuropathic pain. We report here that NaV1.6 contributes up to 60% of the TTX-S current in large, and 34% in small DRG neurons. We also show NaV1.6 accumulates at nodes of Ranvier within the neuroma following spared nerve injury (SNI). Although NaV1.8-Cre driven NaV1.6 knockout does not alter acute, infammatory or neuropathic pain behaviors, AAV-Cre mediated NaV1.6 knockout in adult mice partially attenuates SNI-induced mechanical allodynia. Additionally, AAV-Cre mediated NaV1.6 knockout, mostly in large DRG neurons, signifcantly attenuates excitability of these neurons after SNI and reduces NaV1.6 accumulation at nodes of Ranvier at the neuroma. -
The Action Potential
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/6316219 The action potential ARTICLE in PRACTICAL NEUROLOGY · JULY 2007 Source: PubMed CITATIONS READS 16 64 2 AUTHORS, INCLUDING: Mark W Barnett The University of Edinburgh 21 PUBLICATIONS 661 CITATIONS SEE PROFILE Available from: Mark W Barnett Retrieved on: 24 October 2015 192 Practical Neurology HOW TO UNDERSTAND IT Pract Neurol 2007; 7: 192–197 The action potential Mark W Barnett, Philip M Larkman t is over 60 years since Hodgkin and called ion channels that form the permeation Huxley1 made the first direct recording of pathways across the neuronal membrane. the electrical changes across the neuro- Although the first electrophysiological nal membrane that mediate the action recordings from individual ion channels were I 2 potential. Using an electrode placed inside a not made until the mid 1970s, Hodgkin and squid giant axon they were able to measure a Huxley predicted many of the properties now transmembrane potential of around 260 mV known to be key components of their inside relative to outside, under resting function: ion selectivity, the electrical basis conditions (this is called the resting mem- of voltage-sensitivity and, importantly, a brane potential). The action potential is a mechanism for quickly closing down the transient (,1 millisecond) reversal in the permeability pathways to ensure that the polarity of this transmembrane potential action potential only moves along the axon in which then moves from its point -

Week 5 Lecture Slides (PDF)
Why Does Cocaine Cause Fatal Heart Attacks? • Mental stimulation: Cocaine encourages vigorous activity • Sympathetic stimulation: Cocaine speeds heart rate directly • Vasoconstriction: Impairs blood flow to heart • Numbing: Blocks signals in heart 1 The Action Potential • How does it work? • Epilepsy and anticonvulsants • Local anesthetics (e.g. Novocaine) • Alcohol and alcohol antagonists • Shock therapies 2 Don’t worry • Don’t worry – this is the most confusing, complex, and technical bit of the whole class. I promise we won’t do this again. • This is important, so please pay attention and ask questions whenever you don’t understand. 3 Plan of action: • Electric cells? What? • How batteries work • The four batteries inside every neuron • Channels opening and closing • Putting it all together: The Action Potential 4 The potassium battery (The resting potential) • Draw it on the board (outside cell on top, draw leak channels) • Which way does the diffusion force point? • Which way does the electric force point? • What equilibrium does it eventually settle into? 5 Ion concentrations in and around axons: Concentration Concentration Ratio E (at 37 C, Ion ion outside (in mM) inside (in mM) Out:In in mV) K+ 5 100 1 : 20 -80 Na+ 150 15 10 : 1 +62 10,000 : Ca++ 2 0.0002 1 +123 Cl- 150 13 11.5 : 1 -65 6 What happens if we open and close channels? • Whenever you open a channel, you pull the voltage CLOSER to that channel’s REVERSAL POTENTIAL. The reversal potential is simply the Nernst potential for the one ion in question if only one ion passes through the channel. -

Saxitoxin and Tetrodotoxin. Electrostatic Effects on Sodium Channel Gating Current in Crayfish Axons
SAXITOXIN AND TETRODOTOXIN. ELECTROSTATIC EFFECTS ON SODIUM CHANNEL GATING CURRENT IN CRAYFISH AXONS STEVEN T. HEGOENESS AND JOHN G. STARKUS Department ofPhysiology, John A. Burns School ofMedicine, and the Bekesy Laboratory of Neurobiology, Pacific Biomedical Research Center, University ofHawaii, Honolulu, Hawaii 96822 ABSTRACT The effects of extracellular saxitoxin (STX) and tetrodotoxin (TTX) on gating current (I,ON) were studied in voltage clamped crayfish giant axons. At a holding potential (VH) of -90 mV, integrated gating charge (QON) was found to be 56% suppressed when 200 nM STX was added to the external solution, and 75% suppressed following the addition of 200 nM TTX. These concentrations of toxin are sufficiently high to block >99% of sodium channels. A smaller suppression of ION was observed when 1 nM STX was used (KD- 1-2 nM STX). The suppression of I,ON by external toxin was found to be hold potential dependent, with only minimal suppression observed at the most hyperpolarized hold potentials, - 140 to - 120 mV. The maximal effect of these toxins on ION was observed at hold potentials where the QON vs. VH plot was found to be steepest, -100 to -80 mV. The suppression of ION induced by TTX is partially relieved following the removal of fast inactivation by intracellular treatment with N-bromoacetamide (NBA). The effect of STX and TTX on IgON is equivalent to a hyperpolarizing shift in the steady state inactivation curve, with 200 nM STX and 200 nM TTX inducing shifts of4.9 ± 1.7 mV and 10.0 ± 2.1 mV, respectively. Our results are consistent with a model where the binding of toxin displaces a divalent cation from a negatively charged site near the external opening of the sodium channel, thereby producing a voltage offset sensed by the channel gating apparatus. -

Acid-Sensing Ion Channel 3 Matches the Acid-Gated Current in Cardiac Ischemia-Sensing Neurons
Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons Stephani P. Sutherland*, Christopher J. Benson, John P. Adelman, and Edwin W. McCleskey The Vollum Institute, Oregon Health Sciences University, Portland, OR 97201-3098 Edited by Denis Baylor, Stanford University School of Medicine, Stanford, CA, and approved October 26, 2000 (received for review August 23, 2000) Cardiac afferents are sensory neurons that mediate angina, pain ically (about 10-fold) greater amplitude in the sympathetics (7). The that occurs when the heart receives insufficient blood supply for its currents are blocked by amiloride and pass Naϩ better than Kϩ. metabolic demand (ischemia). These neurons display enormous This behavior of the currents identifies them as being carried by acid-evoked depolarizing currents, and they fire action potentials acid-sensing ion channels (ASICs) and distinguishes them from in response to extracellular acidification that accompanies myo- another type of proton-gated channel, vanilloid receptors. Krish- ϩ cardial ischemia. Here we show that acid-sensing ion channel 3 tal’s group identified ASICs as several kinetically distinct, Na - ϩ (ASIC3), but no other known acid-sensing ion channel, reproduces selective, Ca2 -permeant currents in sensory neurons that are the functional features of the channel that underlies the large evoked by lowered pH and are blocked by amiloride (13–16). acid-evoked current in cardiac afferents. ASIC3 and the native Lazdunski’s group found that the currents are carried by a proton- sensitive subfamily of channels within the larger family of epithelial channel are both especially sensitive to pH, interact similarly with ϩ Ca2؉, and gate rapidly between closed, open, and desensitized Na channels and degenerins of Caenorhabditis elegans (17). -

Membrane Potentials As Signals
Membrane Potentials as Signals The membrane potential allows a cell to function as a battery, providing electrical power to activities within the cell and between cells. KEY POINTS A membrane potential is the difference in electrical potential between the interior and the exterior of a biological cell. In electrically excitable cells, changes in membrane potential are used for transmitting signals within the cell. The opening and closing of ion channels can induce changes from the resting potential. Depolarization is when the interior voltage becomes more positive and hyperpolarization when it becomes more negative. TERMS Graded potentials Graded potentials vary in size and arise from the summation of the individual actions of ligand- gated ion channel proteins, and decrease over time and space. Depolarization Depolarization is a change in a cell's membrane potential, making it more positive, or less negative, and may result in generation of an action potential. Action potential A short term change in the electrical potential that travels along a cell such as a nerve or muscle fiber. EXAMPLE In neurons, a sufficiently large depolarization can evoke an action potential in which the membrane potential changes rapidly. Give us feedback on this content: Membrane potential (also transmembrane potential or membrane voltage) is the difference in electrical potential between the interior and the exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions.