ATP-Sensitive Potassium Channelopathies: Focus on Insulin Secretion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nhbs Annual New and Forthcoming Titles Issue: 2000 Complete January 2001 [email protected] +44 (0)1803 865913
nhbs annual new and forthcoming titles Issue: 2000 complete January 2001 [email protected] +44 (0)1803 865913 The NHBS Monthly Catalogue in a complete yearly edition Zoology: Mammals Birds Welcome to the Complete 2000 edition of the NHBS Monthly Catalogue, the ultimate Reptiles & Amphibians buyer's guide to new and forthcoming titles in natural history, conservation and the Fishes environment. With 300-400 new titles sourced every month from publishers and research organisations around the world, the catalogue provides key bibliographic data Invertebrates plus convenient hyperlinks to more complete information and nhbs.com online Palaeontology shopping - an invaluable resource. Each month's catalogue is sent out as an HTML Marine & Freshwater Biology email to registered subscribers (a plain text version is available on request). It is also General Natural History available online, and offered as a PDF download. Regional & Travel Please see our info page for more details, also our standard terms and conditions. Botany & Plant Science Prices are correct at the time of publication, please check www.nhbs.com for the Animal & General Biology latest prices. Evolutionary Biology Ecology Habitats & Ecosystems Conservation & Biodiversity Environmental Science Physical Sciences Sustainable Development Data Analysis Reference Mammals Activity Patterns in Small Mammals 318 pages | 59 figs, 11 tabs | Springer An Ecological Approach Hbk | 2000 | 354059244X | #109391A | Edited by S Halle and NC Stenseth £100.00 BUY Links chronobiology with behavioural and evolutionary ecology, drawing on research on mammals ranging from mongooses and civets to weasels, martens and shrews. .... African Rhino 92 pages | B/w photos, figs, tabs | IUCN Status Survey and Conservation Action Plan Pbk | 1999 | 2831705029 | #106031A | Richard Emslie and Martin Brooks £15.00 BUY Action plan aimed at donors, government and non-government organisations, and all those involved in rhino conservation. -
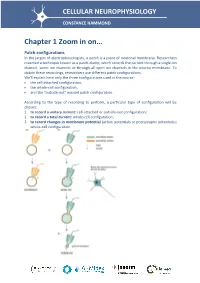
Chapter 1 Zoom in On… Patch Configurations in the Jargon of Electrophysiologists, a Patch Is a Piece of Neuronal Membrane
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 1 Zoom in on… Patch configurations In the jargon of electrophysiologists, a patch is a piece of neuronal membrane. Researchers invented a technique known as a patch-clamp, which records the current through a single ion channel, some ion channels or through all open ion channels in the neuron membrane. To obtain these recordings, researchers use different patch configurations. We'll explain here only the three configurations used in the course: the cell-attached configuration, the whole-cell configuration, and the "outside-out" excised patch configuration. According to the type of recording to perform, a particular type of configuration will be chosen: 1. to record a unitary current: cell-attached or outside-out configuration; 2. to record a total current: whole-cell configuration; 3. to record changes in membrane potential (action potentials or postsynaptic potentials): whole-cell configuration. The cell-attached configuration (attached to the pipette - Figure a) First, the pipette is filled with an extracellular fluid. Positive pressure is applied in the electrode by means of a syringe connected to the electrode, so that the intrapipette fluid tends to leave the pipette. The electrode is then brought near to the soma of a neuron. When the electrode touches the membrane, the positive pressure is withdrawn to draw the membrane toward the mouth of the pipette. We wait without moving the pipette; a seal is made between the walls of the pipette and the soma membrane. This seal strength can be measured by applying a low level of amplitude current in the pipette. Since V = RI, one can measure the resistance of the seal. -

Female Fellows of the Royal Society
Female Fellows of the Royal Society Professor Jan Anderson FRS [1996] Professor Ruth Lynden-Bell FRS [2006] Professor Judith Armitage FRS [2013] Dr Mary Lyon FRS [1973] Professor Frances Ashcroft FMedSci FRS [1999] Professor Georgina Mace CBE FRS [2002] Professor Gillian Bates FMedSci FRS [2007] Professor Trudy Mackay FRS [2006] Professor Jean Beggs CBE FRS [1998] Professor Enid MacRobbie FRS [1991] Dame Jocelyn Bell Burnell DBE FRS [2003] Dr Philippa Marrack FMedSci FRS [1997] Dame Valerie Beral DBE FMedSci FRS [2006] Professor Dusa McDuff FRS [1994] Dr Mariann Bienz FMedSci FRS [2003] Professor Angela McLean FRS [2009] Professor Elizabeth Blackburn AC FRS [1992] Professor Anne Mills FMedSci FRS [2013] Professor Andrea Brand FMedSci FRS [2010] Professor Brenda Milner CC FRS [1979] Professor Eleanor Burbidge FRS [1964] Dr Anne O'Garra FMedSci FRS [2008] Professor Eleanor Campbell FRS [2010] Dame Bridget Ogilvie AC DBE FMedSci FRS [2003] Professor Doreen Cantrell FMedSci FRS [2011] Baroness Onora O'Neill * CBE FBA FMedSci FRS [2007] Professor Lorna Casselton CBE FRS [1999] Dame Linda Partridge DBE FMedSci FRS [1996] Professor Deborah Charlesworth FRS [2005] Dr Barbara Pearse FRS [1988] Professor Jennifer Clack FRS [2009] Professor Fiona Powrie FRS [2011] Professor Nicola Clayton FRS [2010] Professor Susan Rees FRS [2002] Professor Suzanne Cory AC FRS [1992] Professor Daniela Rhodes FRS [2007] Dame Kay Davies DBE FMedSci FRS [2003] Professor Elizabeth Robertson FRS [2003] Professor Caroline Dean OBE FRS [2004] Dame Carol Robinson DBE FMedSci -

Helping Diabetes Sufierers
Helping diabetes sufferers Thanks to work carried out by researchers at the University of Oxford, patients with a particular genetic form of diabetes can now be treated with an oral tablet rather than an insulin injection, which dramatically improves their blood glucose control and quality of life. www.ox.ac.uk/oxfordimpacts In the past, people with neonatal diabetes faced a life of insulin injections. However, the discovery that their diabetes is due to activating mutations in the potassium channel has enabled them to switch to sulphonylurea tablet therapy. This has not only enhanced their blood sugar control and lowered their risk of developing the secondary complications of diabetes; it has also improved their quality of life and that of their families. In a few rare cases, patients with specific genetic defects not only have diabetes, they also show developmental delay, and start to walk and talk much later than expected for their age. Treatment with sulphonylurea tablets can often help these problems too. These patients have neonatal diabetes, a rare Professors Ashcroft and Hattersley organised the type of diabetes that usually develops within six first meeting for patients with neonatal diabetes months of birth. It results from a genetic defect and their families in July 2009, which enabled in a protein known as a potassium channel, which them to meet one another as well as the scientists is crucially important for ensuring that insulin is involved in the research. secreted when blood sugar levels rise. Professor Frances Ashcroft from the Department of Physiology, Anatomy and Genetics was the first to discover the potassium channel and to show how it controls insulin secretion. -

Nernst Potentials and Membrane Potential Changes
UNDERSTANDING MEMBRANE POTENTIAL CHANGES IN TERMS OF NERNST POTENTIALS: For seeing how a change in conductance to ions affects the membrane potential, follow these steps: 1. Make a graph with membrane potential on the vertical axis (-100 to +55) and time on the horizontal axis. 2. Draw dashed lines indicating the standard Nernst potential (equilibrium potential) for each ion: Na+ = +55 mV, K+ = -90mV, Cl- = -65 mV. 3. Draw lines below the horizontal axis showing the increased conductance to individual ions. 4. Start plotting the membrane potential on the left. Most graphs will start at resting potential (-70 mV) 5. When current injection (Stim) is present, move the membrane potential upward to Firing threshold. 6. For the time during which membrane conductance to a particular ion increases, move the membrane potential toward the Nernst potential for that ion. 7. During the time when conductance to a particular ion decreases, move the membrane potential away from the Nernst potential of that ion, toward a position which averages the conductances of the other ions. 8. When conductances return to their original value, membrane potential will go to its starting value. +55mV ACTION POTENTIAL SYNAPTIC POTENTIALS 0mV Membrane potential Firing threshold Firing threshold -65mV -90mV Time Time Na+ K+ Conductances Stim CHANGES IN MEMBRANE POTENTIAL ALLOW NEURONS TO COMMUNICATE The membrane potential of a neuron can be measured with an intracellular electrode. This 1 provides a measurement of the voltage difference between the inside of the cell and the outside. When there is no external input, the membrane potential will usually remain at a value called the resting potential. -

Sulfonylurea Stimulation of Insulin Secretion Peter Proks,1 Frank Reimann,2 Nick Green,1 Fiona Gribble,2 and Frances Ashcroft1
Sulfonylurea Stimulation of Insulin Secretion Peter Proks,1 Frank Reimann,2 Nick Green,1 Fiona Gribble,2 and Frances Ashcroft1 Sulfonylureas are widely used to treat type 2 diabetes smooth, and skeletal muscle, and some brain neurones. In because they stimulate insulin secretion from pancre- all these tissues, opening of KATP channels in response to atic -cells. They primarily act by binding to the SUR metabolic stress leads to inhibition of electrical activity. subunit of the ATP-sensitive potassium (KATP) channel Thus they are involved in the response to both cardiac and and inducing channel closure. However, the channel is cerebral ischemia (2). They are also important in neuronal still able to open to a limited extent when the drug is regulation of glucose homeostasis (3), seizure protection bound, so that high-affinity sulfonylurea inhibition is not complete, even at saturating drug concentrations. (4), and the control of vascular smooth muscle tone (and, thereby, blood pressure) (5). KATP channels are also found in cardiac, skeletal, and smooth muscle, but in these tissues are composed of The KATP channel is a hetero-octameric complex of two different SUR subunits that confer different drug different types of protein subunits: an inwardly rectifying sensitivities. Thus tolbutamide and gliclazide block Kϩ channel, Kir6.x, and a sulfonylurea receptor, SUR (6,7).  ϩ channels containing SUR1 ( -cell type), but not SUR2 Kir6.x belongs to the family of inwardly rectifying K (cardiac, smooth muscle types), whereas glibenclamide, (Kir) channels and assembles as a tetramer to form the glimepiride, repaglinide, and meglitinide block both types of channels. -

Effect of Hyperkalemia on Membrane Potential: Depolarization
❖ CASE 3 A 6-year-old boy is brought to the family physician after his parents noticed that he had difficulty moving his arms and legs after a soccer game. About 10 minutes after leaving the field, the boy became so weak that he could not stand for about 30 minutes. Questioning revealed that he had complained of weakness after eating bananas, had frequent muscle spasms, and occasionally had myotonia, which was expressed as difficulty in releasing his grip or diffi- culty opening his eyes after squinting into the sun. After a thorough physical examination, the boy was diagnosed with hyperkalemic periodic paralysis. The family was advised to feed the boy carbohydrate-rich, low-potassium foods, give him glucose-containing drinks during attacks, and have him avoid strenuous exercise and fasting. ◆ What is the effect of hyperkalemia on cell membrane potential? ◆ What is responsible for the repolarizing phase of an action potential? ◆ What is the effect of prolonged depolarization on the skeletal muscle Na+ channel? 32 CASE FILES: PHYSIOLOGY ANSWERS TO CASE 3: ACTION POTENTIAL Summary: A 6-year-old boy who experiences profound weakness after exer- cise is diagnosed with hyperkalemic periodic paralysis. ◆ Effect of hyperkalemia on membrane potential: Depolarization. ◆ Repolarization mechanisms: Activation of voltage-gated K+ conductance and inactivation of Na+ conductance. ◆ Effect of prolonged depolarization: Inactivation of Na+ channels. CLINICAL CORRELATION Hyperkalemic periodic paralysis (HyperPP) is a dominant inherited trait caused by a mutation in the α subunit of the skeletal muscle Na+ channel. It occurs in approximately 1 in 100,000 people and is more common and more severe in males. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -
The Action Potential
See discussions, stats, and author profiles for this publication at: http://www.researchgate.net/publication/6316219 The action potential ARTICLE in PRACTICAL NEUROLOGY · JULY 2007 Source: PubMed CITATIONS READS 16 64 2 AUTHORS, INCLUDING: Mark W Barnett The University of Edinburgh 21 PUBLICATIONS 661 CITATIONS SEE PROFILE Available from: Mark W Barnett Retrieved on: 24 October 2015 192 Practical Neurology HOW TO UNDERSTAND IT Pract Neurol 2007; 7: 192–197 The action potential Mark W Barnett, Philip M Larkman t is over 60 years since Hodgkin and called ion channels that form the permeation Huxley1 made the first direct recording of pathways across the neuronal membrane. the electrical changes across the neuro- Although the first electrophysiological nal membrane that mediate the action recordings from individual ion channels were I 2 potential. Using an electrode placed inside a not made until the mid 1970s, Hodgkin and squid giant axon they were able to measure a Huxley predicted many of the properties now transmembrane potential of around 260 mV known to be key components of their inside relative to outside, under resting function: ion selectivity, the electrical basis conditions (this is called the resting mem- of voltage-sensitivity and, importantly, a brane potential). The action potential is a mechanism for quickly closing down the transient (,1 millisecond) reversal in the permeability pathways to ensure that the polarity of this transmembrane potential action potential only moves along the axon in which then moves from its point -

Smutty Alchemy
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2021-01-18 Smutty Alchemy Smith, Mallory E. Land Smith, M. E. L. (2021). Smutty Alchemy (Unpublished doctoral thesis). University of Calgary, Calgary, AB. http://hdl.handle.net/1880/113019 doctoral thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY Smutty Alchemy by Mallory E. Land Smith A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN ENGLISH CALGARY, ALBERTA JANUARY, 2021 © Mallory E. Land Smith 2021 MELS ii Abstract Sina Queyras, in the essay “Lyric Conceptualism: A Manifesto in Progress,” describes the Lyric Conceptualist as a poet capable of recognizing the effects of disparate movements and employing a variety of lyric, conceptual, and language poetry techniques to continue to innovate in poetry without dismissing the work of other schools of poetic thought. Queyras sees the lyric conceptualist as an artistic curator who collects, modifies, selects, synthesizes, and adapts, to create verse that is both conceptual and accessible, using relevant materials and techniques from the past and present. This dissertation responds to Queyras’s idea with a collection of original poems in the lyric conceptualist mode, supported by a critical exegesis of that work. -

Women Physiologists
Women physiologists: Centenary celebrations and beyond physiologists: celebrations Centenary Women Hodgkin Huxley House 30 Farringdon Lane London EC1R 3AW T +44 (0)20 7269 5718 www.physoc.org • journals.physoc.org Women physiologists: Centenary celebrations and beyond Edited by Susan Wray and Tilli Tansey Forewords by Dame Julia Higgins DBE FRS FREng and Baroness Susan Greenfield CBE HonFRCP Published in 2015 by The Physiological Society At Hodgkin Huxley House, 30 Farringdon Lane, London EC1R 3AW Copyright © 2015 The Physiological Society Foreword copyright © 2015 by Dame Julia Higgins Foreword copyright © 2015 by Baroness Susan Greenfield All rights reserved ISBN 978-0-9933410-0-7 Contents Foreword 6 Centenary celebrations Women in physiology: Centenary celebrations and beyond 8 The landscape for women 25 years on 12 "To dine with ladies smelling of dog"? A brief history of women and The Physiological Society 16 Obituaries Alison Brading (1939-2011) 34 Gertrude Falk (1925-2008) 37 Marianne Fillenz (1924-2012) 39 Olga Hudlická (1926-2014) 42 Shelagh Morrissey (1916-1990) 46 Anne Warner (1940–2012) 48 Maureen Young (1915-2013) 51 Women physiologists Frances Mary Ashcroft 56 Heidi de Wet 58 Susan D Brain 60 Aisah A Aubdool 62 Andrea H. Brand 64 Irene Miguel-Aliaga 66 Barbara Casadei 68 Svetlana Reilly 70 Shamshad Cockcroft 72 Kathryn Garner 74 Dame Kay Davies 76 Lisa Heather 78 Annette Dolphin 80 Claudia Bauer 82 Kim Dora 84 Pooneh Bagher 86 Maria Fitzgerald 88 Stephanie Koch 90 Abigail L. Fowden 92 Amanda Sferruzzi-Perri 94 Christine Holt 96 Paloma T. Gonzalez-Bellido 98 Anne King 100 Ilona Obara 102 Bridget Lumb 104 Emma C Hart 106 Margaret (Mandy) R MacLean 108 Kirsty Mair 110 Eleanor A. -

Membrane Potentials As Signals
Membrane Potentials as Signals The membrane potential allows a cell to function as a battery, providing electrical power to activities within the cell and between cells. KEY POINTS A membrane potential is the difference in electrical potential between the interior and the exterior of a biological cell. In electrically excitable cells, changes in membrane potential are used for transmitting signals within the cell. The opening and closing of ion channels can induce changes from the resting potential. Depolarization is when the interior voltage becomes more positive and hyperpolarization when it becomes more negative. TERMS Graded potentials Graded potentials vary in size and arise from the summation of the individual actions of ligand- gated ion channel proteins, and decrease over time and space. Depolarization Depolarization is a change in a cell's membrane potential, making it more positive, or less negative, and may result in generation of an action potential. Action potential A short term change in the electrical potential that travels along a cell such as a nerve or muscle fiber. EXAMPLE In neurons, a sufficiently large depolarization can evoke an action potential in which the membrane potential changes rapidly. Give us feedback on this content: Membrane potential (also transmembrane potential or membrane voltage) is the difference in electrical potential between the interior and the exterior of a biological cell. All animal cells are surrounded by a plasma membrane composed of a lipid bilayer with a variety of types of proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions.