Power Sources Challenge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On the History of the Radiation Reaction1 Kirk T
On the History of the Radiation Reaction1 Kirk T. McDonald Joseph Henry Laboratories, Princeton University, Princeton, NJ 08544 (May 6, 2017; updated March 18, 2020) 1 Introduction Apparently, Kepler considered the pointing of comets’ tails away from the Sun as evidence for radiation pressure of light [2].2 Following Newton’s third law (see p. 83 of [3]), one might suppose there to be a reaction of the comet back on the incident light. However, this theme lay largely dormant until Poincar´e (1891) [37, 41] and Planck (1896) [46] discussed the effect of “radiation damping” on an oscillating electric charge that emits electromagnetic radiation. Already in 1892, Lorentz [38] had considered the self force on an extended, accelerated charge e, finding that for low velocity v this force has the approximate form (in Gaussian units, where c is the speed of light in vacuum), independent of the radius of the charge, 3e2 d2v 2e2v¨ F = = . (v c). (1) self 3c3 dt2 3c3 Lorentz made no connection at the time between this force and radiation, which connection rather was first made by Planck [46], who considered that there should be a damping force on an accelerated charge in reaction to its radiation, and by a clever transformation arrived at a “radiation-damping” force identical to eq. (1). Today, Lorentz is often credited with identifying eq. (1) as the “radiation-reaction force”, and the contribution of Planck is seldom acknowledged. This note attempts to review the history of thoughts on the “radiation reaction”, which seems to be in conflict with the brief discussions in many papers and “textbooks”.3 2 What is “Radiation”? The “radiation reaction” would seem to be a reaction to “radiation”, but the concept of “radiation” is remarkably poorly defined in the literature. -

Classical Mechanics
Classical Mechanics Hyoungsoon Choi Spring, 2014 Contents 1 Introduction4 1.1 Kinematics and Kinetics . .5 1.2 Kinematics: Watching Wallace and Gromit ............6 1.3 Inertia and Inertial Frame . .8 2 Newton's Laws of Motion 10 2.1 The First Law: The Law of Inertia . 10 2.2 The Second Law: The Equation of Motion . 11 2.3 The Third Law: The Law of Action and Reaction . 12 3 Laws of Conservation 14 3.1 Conservation of Momentum . 14 3.2 Conservation of Angular Momentum . 15 3.3 Conservation of Energy . 17 3.3.1 Kinetic energy . 17 3.3.2 Potential energy . 18 3.3.3 Mechanical energy conservation . 19 4 Solving Equation of Motions 20 4.1 Force-Free Motion . 21 4.2 Constant Force Motion . 22 4.2.1 Constant force motion in one dimension . 22 4.2.2 Constant force motion in two dimensions . 23 4.3 Varying Force Motion . 25 4.3.1 Drag force . 25 4.3.2 Harmonic oscillator . 29 5 Lagrangian Mechanics 30 5.1 Configuration Space . 30 5.2 Lagrangian Equations of Motion . 32 5.3 Generalized Coordinates . 34 5.4 Lagrangian Mechanics . 36 5.5 D'Alembert's Principle . 37 5.6 Conjugate Variables . 39 1 CONTENTS 2 6 Hamiltonian Mechanics 40 6.1 Legendre Transformation: From Lagrangian to Hamiltonian . 40 6.2 Hamilton's Equations . 41 6.3 Configuration Space and Phase Space . 43 6.4 Hamiltonian and Energy . 45 7 Central Force Motion 47 7.1 Conservation Laws in Central Force Field . 47 7.2 The Path Equation . -

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

Physics 101 Today Chapter 5: Newton's Third
Physics 101 Today Chapter 5: Newton’s Third Law First, let’s clarify notion of a force : Previously defined force as a push or pull. Better to think of force as an interaction between two objects. You can’t push anything without it pushing back on you ! Whenever one object exerts a force on a second object, the second object exerts an equal and opposite force on the first. Newton’s 3 rd Law - often called “action-reaction ” Eg. Leaning against a wall. You push against the wall. The wall is also pushing on you, equally hard – normal/support force. Now place a piece of paper between the wall and hand. Push on it – it doesn’t accelerate must be zero net force. The wall is pushing equally as hard (normal force) on the paper in the opposite direction to your hand, resulting in zero Fnet . This is more evident when hold a balloon against the wall – it is squashed on both sides. Eg. You pull on a cart. It accelerates. The cart pulls back on you (you feel the rope get tighter). Can call your pull the “ action ” and cart’s pull the “ reaction ”. Or, the other way around. • Newton’s 3 rd law means that forces always come in action -reaction pairs . It doesn’t matter which is called the action and which is called the reaction. • Note: Action-reaction pairs never act on the same object Examples of action-reaction force pairs In fact it is the road’s push that makes the car go forward. Same when we walk – push back on floor, floor pushes us forward. -

HW 6, Question 1: Forces in a Rotating Frame
HW 6, Question 1: Forces in a Rotating Frame Consider a solid platform, like a \merry-go round", that rotates with respect to an inertial frame of reference. It is oriented in the xy plane such that the axis of rotation is in the z-direction, i.e. ! = !z^, which we can take to point out of the page. Now, let us also consider a person walking on the rotating platform, such that this person is walking radially outward on the platform with respect to their (non-inertial) reference frame with velocity v = vr^. In what follows for Question 1, directions in the non-inertial reference frame (i.e. the frame of the walking person) are defined in the following manner: the x0-direction points directly outward from the center of the merry-go round; the y0 points in the tangential direction of rotation in the plane of the platform; and the z0-direction points out of the page, and in the same direction as the z-direction for the inertial reference frame. (a) Identify all forces. Since the person's reference frame is non-inertial, we need to account for the various fictitious forces given the description above, as well as any other external/reaction forces. The angular rate of rotation is constant, and so the Euler force Feul = m(!_ × r) = ~0. The significant forces acting on the person are: 0 • the force due to gravity, Fg = mg = mg(−z^ ), 2 0 • the (fictitious) centrifugal force, Fcen = m! × (! × r) = m! rx^ , 0 • the (fictitious) Coriolis force, Fcor = 2mv × ! = 2m!v(−y^ ), • the normal force the person experiences from the rotating platform N = Nz^0, and • a friction force acting on the person, f, in the x0y0 plane. -

Unique Properties of Water!
Name: _______ANSWER KEY_______________ Class: _____ Date: _______________ Unique Properties of Water! Word Bank: Adhesion Evaporation Polar Surface tension Cohesion Freezing Positive Universal solvent Condensation Melting Sublimation Dissolve Negative 1. The electrons are not shared equally between the hydrogen and oxygen atoms of water creating a Polar molecule. 2. The polarity of water allows it to dissolve most substances. Because of this it is referred to as the universal solvent 3. Water molecules stick to other water molecules. This property is called cohesion. 4. Hydrogen bonds form between adjacent water molecules because the positive charged hydrogen end of one water molecule attracts the negative charged oxygen end of another water molecule. 5. Water molecules stick to other materials due to its polar nature. This property is called adhesion. 6. Hydrogen bonds hold water molecules closely together which causes water to have high surface tension. This is why water tends to clump together to form drops rather than spread out into a thin film. 7. Condensation is when water changes from a gas to a liquid. 8. Sublimation is when water changes from a solid directly to a gas. 9. Freezing is when water changes from a liquid to a solid. 10. Melting is when water changes from a solid to a liquid. 11. Evaporation is when water changes from a liquid to a gas. 12. Why does ice float? Water expands as it freezes, so it is LESS DENSE AS A SOLID. 13. What property refers to water molecules resembling magnets? How are these alike? Polar bonds create positive and negative ends of the molecule. -

Lithium Hydride Powered PEM Fuel Cells for Long-Duration Small Mobile Robotic Missions
Lithium Hydride Powered PEM Fuel Cells for Long-Duration Small Mobile Robotic Missions Jekanthan Thangavelautham, Daniel Strawser, Mei Yi Cheung Steven Dubowsky Abstract— This paper reports on a study to develop power supplies for small mobile robots performing long duration missions. It investigates the use of fuel cells to achieve this objective, and in particular Proton Exchange Membrane (PEM) fuel cells. It is shown through a representative case study that, in theory, fuel cell based power supplies will provide much longer range than the best current rechargeable battery technology. It also briefly discusses an important limitation that prevents fuel cells from achieving their ideal performance, namely a practical method to store their fuel (hydrogen) in a form that is compatible with small mobile field robots. A very efficient Fig. 1. Example of small robots (Left) A ball shaped hopping robot concept fuel storage concept based on water activated lithium hydride for exploration of extreme terrains and caves developed for NASA. (Right) (LiH) is proposed that releases hydrogen on demand. This iRobot 110 Firstlook used for observation, security and search and rescue. concept is very attractive because water vapor from the air is passively extracted or waste water from the fuel cell is recycled and transferred to the lithium hydride where the hydrogen is the near future. Hence, new means for powering field robots “stripped” from water and is returned to the fuel cell to form more water. This results in higher hydrogen storage efficiencies need to be considered. than conventional storage methods. Experimental results are This paper explores the use of fuel cells, and in particular presented that demonstrate the effectiveness of the approach. -

A Short History of Physics (Pdf)
A Short History of Physics Bernd A. Berg Florida State University PHY 1090 FSU August 28, 2012. References: Most of the following is copied from Wikepedia. Bernd Berg () History Physics FSU August 28, 2012. 1 / 25 Introduction Philosophy and Religion aim at Fundamental Truths. It is my believe that the secured part of this is in Physics. This happend by Trial and Error over more than 2,500 years and became systematic Theory and Observation only in the last 500 years. This talk collects important events of this time period and attaches them to the names of some people. I can only give an inadequate presentation of the complex process of scientific progress. The hope is that the flavor get over. Bernd Berg () History Physics FSU August 28, 2012. 2 / 25 Physics From Acient Greek: \Nature". Broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves. The universe is commonly defined as the totality of everything that exists or is known to exist. In many ways, physics stems from acient greek philosophy and was known as \natural philosophy" until the late 18th century. Bernd Berg () History Physics FSU August 28, 2012. 3 / 25 Ancient Physics: Remarkable people and ideas. Pythagoras (ca. 570{490 BC): a2 + b2 = c2 for rectangular triangle: Leucippus (early 5th century BC) opposed the idea of direct devine intervention in the universe. He and his student Democritus were the first to develop a theory of atomism. Plato (424/424{348/347) is said that to have disliked Democritus so much, that he wished his books burned. -

Hydrogen, Nitrogen and Argon Chemistry
Hydrogen, Nitrogen and Argon Chemistry Author: Brittland K. DeKorver Institute for Chemical Education and Nanoscale Science and Engineering Center University of Wisconsin-Madison Purpose: To learn about 3 of the gases that are in Earth’s atmosphere. Learning Objectives: 1. Understand that the atmosphere is made up of many gases. 2. Learn about the differences in flammability of nitrogen and hydrogen. 3. Learn what is produced during electrolysis. Next Generation Science Standards (est. 2013): PS1.A: Structure and Properties of Matter PS1.B: Chemical Reactions PS3.D: Energy in Chemical Processes and Everyday Life (partial) ETS1.A: Defining Engineering Problems ETS1.B: Designing Solutions to Engineering Problems ETS1.C: Optimizing the Design Solution National Science Education Standards (valid 1996-2013): Physical Science Standards: Properties and changes of properties in matter Earth and Space Science Standards: Structure of the earth system Suggested Previous Activities: Oxygen Investigation and Carbon Dioxide Chemistry Grade Level: 2-8 Time: 1 hour Materials: Safety glasses Manganese dioxide Work gloves (for handling Tea-light candles steel wool) Matches Test tubes Spatulas Beakers Wooden splints 100mL graduated cylinders Waste bucket for burnt 15mL graduated cylinders materials 3% hydrogen peroxide Vinegar Baking soda Safety: 1. Students should wear safety glasses during all activities. 2. Students should not be allowed to use matches or candle. 3. Students should be cautioned to only mix the chemicals as directed. Introduction: Air refers to the mixture of gases that make up the Earth’s atmosphere. In this lesson, students will learn about hydrogen (present in very, very small amounts in our atmosphere), nitrogen (the most prevalent gas), and argon (third behind nitrogen and oxygen.) Procedures: 1. -

MODULE 11: GLOSSARY and CONVERSIONS Cell Engines
Hydrogen Fuel MODULE 11: GLOSSARY AND CONVERSIONS Cell Engines CONTENTS 11.1 GLOSSARY.......................................................................................................... 11-1 11.2 MEASUREMENT SYSTEMS .................................................................................. 11-31 11.3 CONVERSION TABLE .......................................................................................... 11-33 Hydrogen Fuel Cell Engines and Related Technologies: Rev 0, December 2001 Hydrogen Fuel MODULE 11: GLOSSARY AND CONVERSIONS Cell Engines OBJECTIVES This module is for reference only. Hydrogen Fuel Cell Engines and Related Technologies: Rev 0, December 2001 PAGE 11-1 Hydrogen Fuel Cell Engines MODULE 11: GLOSSARY AND CONVERSIONS 11.1 Glossary This glossary covers words, phrases, and acronyms that are used with fuel cell engines and hydrogen fueled vehicles. Some words may have different meanings when used in other contexts. There are variations in the use of periods and capitalization for abbrevia- tions, acronyms and standard measures. The terms in this glossary are pre- sented without periods. ABNORMAL COMBUSTION – Combustion in which knock, pre-ignition, run- on or surface ignition occurs; combustion that does not proceed in the nor- mal way (where the flame front is initiated by the spark and proceeds throughout the combustion chamber smoothly and without detonation). ABSOLUTE PRESSURE – Pressure shown on the pressure gauge plus at- mospheric pressure (psia). At sea level atmospheric pressure is 14.7 psia. Use absolute pressure in compressor calculations and when using the ideal gas law. See also psi and psig. ABSOLUTE TEMPERATURE – Temperature scale with absolute zero as the zero of the scale. In standard, the absolute temperature is the temperature in ºF plus 460, or in metric it is the temperature in ºC plus 273. Absolute zero is referred to as Rankine or r, and in metric as Kelvin or K. -
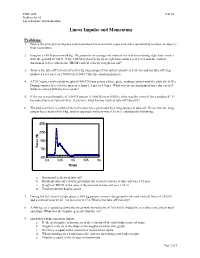
Linear Impulse and Momentum
EXSC 408L Fall '03 Problem Set #5 Linear Impulse and Momentum Linear Impulse and Momentum Problems: 1. Derive the principal of impulse and momentum from an initial cause and effect relationship to show an object’s final momentum. 2. Imagine a 100 N person walking. He generates an average net vertical reaction force during right foot contact with the ground of 100 N. If the TBCM vertical velocity at right foot contact is -0.2 m/s and the contact duration is 0.25 s, what is the TBCM vertical velocity at right toe off? 3. What is the take-off velocity of a 68.0 kg long jumper if his initial velocity is 8.50 m/s and his take-off step produces a net force of 2700 N for 0.200 s? Use the equation from #1. 4. A USC runner with a body weight of 490.5 N runs across a force plate, making contact with the plate for 0.25 s. During contact, her velocity increases from 2.5 m/s to 4.0 m/s. What was the net horizontal force she exerted while in contact with the force plate? 5. If the net vertical impulse of a 580 N person is 1800 Ns over 0.500 s, what was the vertical force produced? If her initial vertical velocity was –0.200 m/s, what was her vertical take-off velocity? 6. The plot seen here is a plot of the horizontal force generated by a long-jumper at take-off. Given that the long- jumper has a mass of 68.0 kg, and his approach velocity was 8.30 m/s, calculate the following: 3750 2750 1750 Force (N) 750 -250 0.4 0.45 0.5 0.55 0.6 Time(s) a. -

Hydrogen and Its Components
HYDROGEN AND ITS COMPONENTS Discovery In 1671, Robert Boyle discovered and described the reaction between iron filings and dilute acids, which resulted in the production of hydrogen gas. In 1766-81, Henry Cavendish was the first to recognize that hydrogen gas was a discrete substance, and that it produced water when burned. He named it "flammable air". In 1783, Antoine Lavoisier gave the element the name hydrogen (from the Greek σδρο-hydro meaning "water" and -γενης genes meaning "creator") when he and Pierre-Simon Laplace reproduced Cavendish's finding that water was produced when hydrogen was burned. Hydrogen was liquefied for the first time by James Dewar in 1898 by using regenerative cooling and his invention, the vacuum flask. He produced solid hydrogen the next year. Deuterium was discovered in December 1931 by Harold Urey, and tritium was prepared in 1934 by Ernest Rutherford, Mark Oliphant, and Paul Harteck. Heavy water, which consists of deuterium in the place of regular hydrogen, was discovered by Urey's group in 1932. The nickel hydrogen battery was used for the first time in 1977 aboard the U.S. Navy's Navigation technology satellite-2 (NTS-2). It had two caesium atomic clocks on board and helped to show that satellite navigation based on precise timing was possible. In the dark part of its orbit, the Hubble Space Telescope is powered by nickel-hydrogen batteries, which were finally replaced in May 2009, more than 19 years after launch, and 13 years passed their design life. from NASA (accessed 2 Feb 2015) Isotopes of hydrogen Hydrogen has three naturally occurring isotopes, denoted 1H, 2H and 3H.