Determine Most Stable Isobar for Nuclides with A= (15-30) & (101- 115)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CME Search Before Isobar Collisions and Blind Analysis from STAR
CME Search Before Isobar Collisions and Blind Analysis From STAR Prithwish Tribedy for the STAR collaboration The 36th Winter Workshop on Nuclear Dynamics 1-7th March, 2020, Puerto Vallarta, Mexico In part supported by Introduction Solenoidal Tracker at RHIC (STAR) RHIC has collided multiple ion species; year 2018 was dedicated to search for effects driven by strong electromagnetic fields by STAR Isobars: Ru+Ru, Zr+Zr @ 200GeV (2018) Low energy: Au+Au 27 GeV (2018) Large systems : U+U, Au+Au @ 200 GeV P.Tribedy, WWND 2 The Chiral Magnetic Effect (Cartoon Picture) Quarks Quarks randomly aligned oriented along B antiquarks I II III IV More More right left handed handed quarks quarks J || B J || -B CME converts chiral imbalance to observable electric current P.Tribedy, WWND 2020 3 New Theory Guidance : Complexity Of An Event Magnetic field map Pb+Pb @ 2.76 TeV Axial charge profile b=11.4 fm, Npart=56 Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 uR>uL 0.2 4 uR<uL 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, Tribedy, Phys. Rev. C 92, Based on: Lappi, Schlichting, Phys. Rev. D 97, 011902 (2015) 034034 (2018) Going beyond cartoon picture: 1) Fluctuations dominate e-by-e physics, 2) B-field & domain size of axial-charge change with √s P.Tribedy, WWND 2020 4 New Theory Guidance : Complexity Of An Event Pb+Pb @ 2.76 TeV Magnetic field b=11.4 fm, N Axial charge profile Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 u 0.2 4 u 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, -

1 11. Nuclear Chemistry 11.1 Stable and Unstable Nuclides Very Large
11. Nuclear Chemistry Chemical reactions occur as a result of loosing/gaining and sharing electrons in the valance shell which is far away from the atomic nucleus as we described in previous chapters in chemical bonding. In chemical reactions identity of the elements (atomic) and the makeup of the nuclei (mass due to protons and neutrons) is preserved which is reflected in the Law of Conservation of mass. This idea of atomic nucleus is always stable was shattered as Henri Becquerel discovered radioactivity in uranium compound where uranium nuclei changes or undergo nuclear reactions where nuclei of an element is transformed into nuclei of different element(s) while emitting ionization radiation. Marie Curie also began a study of radioactivity in a different form of uranium ore called pitchblende and she discovered the existence of two more highly radioactive new elements radium and polonium formed as the products during the decay of unstable nuclide of uranium-235. Curie measure that the radiation emanated was proportional to the amount (moles or number of nuclides) of radioactive element present, and she proposed that radiation was a property nucleus of an unstable atom. The area of chemistry that focuses on the nuclear changes is called nuclear chemistry. What changes in a nuclide result from the loss of each of the following? a) An alpha particle. b) A gamma ray. c) An electron. d) A neutron. e) A proton. Answer: a), c), d), e) 11.1 Stable and Unstable Nuclides There are stable and unstable radioactive nuclides. Unstable nuclides emit subatomic particles, with alpha −α, beta −β, gamma −γ, proton-p, neutrons-n being the most common. -
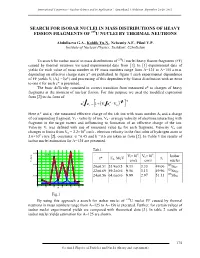
Search for Isobar Nuclei in Mass Distributions of Heavy Fission Fragments of 235U Nuclei by Thermal Neutrons
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 SEARCH FOR ISOBAR NUCLEI IN MASS DISTRIBUTIONS OF HEAVY FISSION FRAGMENTS OF 235U NUCLEI BY THERMAL NEUTRONS Abdullaeva G.A., Koblik Yu.N., Nebesniy A.F., Pikul V.P. Institute of Nuclear Physics, Tashkent, Uzbekistan To search for isobar nuclei in mass distributions of 235U nuclei heavy fission fragments (FF) caused by thermal neutrons we used experimental data from [1]. In [1] experimental data of yields for each value of mass number in FF mass numbers range from A=125 to A=155 a.m.u. depending on effective charge state z* are published. In figure 1 such experimental dependence of FF yields Yi (Ai) =f(z*) and processing of this dependence by Gauss distribution with an error to one σ for each z* is presented. The basic difficulty consisted in correct transition from measured z* to charges of heavy fragments at the moment of nuclear fission. For this purpose we used the modified expression from [2] in the form of: k * α k1 i i ii VzV1zz 0 . Here zi* and zi - the measured effective charge of the i-th ion with mass number Ai and a charge of corresponding fragment, Vi - velocity of ion, Vo - average velocity of electrons interacting with fragment in the target matter and influencing to formation of an effective charge of the ion. Velocity Vi was defined with use of measured value Ek for each fragment. Velocity Vo can 8 changes in limits from Vo = 2.2×10 cm/s - electron velocity in the first orbit of hydrogen atom to 3.6×108 cm/s [2], constants: α =0.45 and k =0.6 are taken as from [2]. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

The Nucleus and Nuclear Instability
Lecture 2: The nucleus and nuclear instability Nuclei are described using the following nomenclature: A Z Element N Z is the atomic number, the number of protons: this defines the element. A is called the “mass number” A = N + Z. N is the number of neutrons (N = A - Z) Nuclide: A species of nucleus of a given Z and A. Isotope: Nuclides of an element (i.e. same Z) with different N. Isotone: Nuclides having the same N. Isobar: Nuclides having the same A. [A handy way to keep these straight is to note that isotope includes the letter “p” (same proton number), isotone the letter “n” (same neutron number), and isobar the letter “a” (same A).] Example: 206 82 Pb124 is an isotope of 208 82 Pb126 is an isotone of 207 83 Bi124 and an isobar of 206 84 Po122 1 Chart of the Nuclides Image removed. 90 natural elements 109 total elements All elements with Z > 42 are man-made Except for technicium Z=43 Promethium Z = 61 More than 800 nuclides are known (274 are stable) “stable” unable to transform into another configuration without the addition of outside energy. “unstable” = radioactive Images removed. [www2.bnl.gov/ton] 2 Nuclear Structure: Forces in the nucleus Coulomb Force Force between two point charges, q, separated by distance, r (Coulomb’s Law) k0 q1q2 F(N) = 9 2 -2 r 2 k0 = 8.98755 x 10 N m C (Boltzman constant) Potential energy (MeV) of one particle relative to the other k q q PE(MeV)= 0 1 2 r Strong Nuclear Force • Acts over short distances • ~ 10-15 m • can overcome Coulomb repulsion • acts on protons and neutrons Image removed. -

Background and Derivation of ANS-5.4 Standard Fission Product Release Model
NUREG/CR-7003 PNNL-18490 Background and Derivation of ANS-5.4 Standard Fission Product Release Model Office of Nuclear Regulatory Research AVAILABILITY OF REFERENCE MATERIALS IN NRC PUBLICATIONS NRC Reference Material Non-NRC Reference Material As of November 1999, you may electronically access Documents available from public and special technical NUREG-series publications and other NRC records at libraries include all open literature items, such as NRC’s Public Electronic Reading Room at books, journal articles, and transactions, Federal http://www.nrc.gov/reading-rm.html. Register notices, Federal and State legislation, and Publicly released records include, to name a few, congressional reports. Such documents as theses, NUREG-series publications; Federal Register notices; dissertations, foreign reports and translations, and applicant, licensee, and vendor documents and non-NRC conference proceedings may be purchased correspondence; NRC correspondence and internal from their sponsoring organization. memoranda; bulletins and information notices; inspection and investigative reports; licensee event reports; and Commission papers and their attachments. Copies of industry codes and standards used in a substantive manner in the NRC regulatory process are NRC publications in the NUREG series, NRC maintained at— regulations, and Title 10, Energy, in the Code of The NRC Technical Library Federal Regulations may also be purchased from one Two White Flint North of these two sources. 11545 Rockville Pike 1. The Superintendent of Documents Rockville, MD 20852–2738 U.S. Government Printing Office Mail Stop SSOP Washington, DC 20402–0001 These standards are available in the library for Internet: bookstore.gpo.gov reference use by the public. Codes and standards are Telephone: 202-512-1800 usually copyrighted and may be purchased from the Fax: 202-512-2250 originating organization or, if they are American 2. -

Status of Fission Product Yield Data for Thermal Reactors
Atomic Energy of Canada Limited STATUS OF FISSION PRODUCT YIELD DATA FOR THERMAL REACTORS by W.H. WALKER Chalk River Nuclear Laboratories Chalk River, Ontario February 1974 AECL-4704, tr - STATUS OF FISSION PRODUCT YIELD DATA FOR THERMAL REACTORS W.H. Walker Review Paper lla for the I.A.E.A. Panel on Fission Product Nuclear Data, held at Bologna, Italy, November 26 - 30, 1973. Chalk River Nuclear Laboratories Chalk River, Ontario February 1974 AECL-4704 STATUS OF FISSION PRODUCT YIELD DATA FOR THERMAL REACTORS by W.H. Walker ABSTRACT Methods of measuring fission product yields are discussed. Measured and evaluated chain yields from thermal neutron fission of 233U, 235U, 239Pu and 21flPu are reviewed and evaluated yields are compared in an appendix. Measured fractional and direct yields of interest in reactor cal- culations are discussed and compiled in an appendix. The few data on cumulative yields from fission by low energy epithermal neutrons are noted and their significance in reactor calculations estimated. Chalk River Nuclear Laboratories Chalk River, Ontario February 1974 AECL-4704 Connaissances actuelles, en matière de rendement des produits de fission, pour les réacteurs thermiques par W.H. Walker Résumé Les méthodes permettant de mesurer le rendement des produits de fission font l'objet de commentaires. Des rendements en chaîne ayant été mesurés et évalués à partir 2 3 3 235 239 de la fission, par neutrons thermiques, de U, U, Pu 241 et Pu sont passés en revue et les rendements évalués font l'objet d'une comparaison dans une annexe. Les rende- ments mesurés, directs ou fractionnels, qui présentent un intérêt pour les calculs de réacteur font l'objet de commen- taires et ils sent compilés dans une annexe. -

The Delimiting/Frontier Lines of the Constituents of Matter∗
The delimiting/frontier lines of the constituents of matter∗ Diógenes Galetti1 and Salomon S. Mizrahi2 1Instituto de Física Teórica, Universidade Estadual Paulista (UNESP), São Paulo, SP, Brasily 2Departamento de Física, CCET, Universidade Federal de São Carlos, São Carlos, SP, Brasilz (Dated: October 23, 2019) Abstract Looking at the chart of nuclides displayed at the URL of the International Atomic Energy Agency (IAEA) [1] – that contains all the known nuclides, the natural and those produced artificially in labs – one verifies the existence of two, not quite regular, delimiting lines between which dwell all the nuclides constituting matter. These lines are established by the highly unstable radionuclides located the most far away from those in the central locus, the valley of stability. Here, making use of the “old” semi-empirical mass formula for stable nuclides together with the energy-time uncertainty relation of quantum mechanics, by a simple calculation we show that the obtained frontier lines, for proton and neutron excesses, present an appreciable agreement with the delimiting lines. For the sake of presenting a somewhat comprehensive panorama of the matter in our Universe and their relation with the frontier lines, we narrate, in brief, what is currently known about the astrophysical nucleogenesis processes. Keywords: chart of nuclides, nucleogenesis, nuclide mass formula, valley/line of stability, energy-time un- certainty relation, nuclide delimiting/frontier lines, drip lines arXiv:1909.07223v2 [nucl-th] 22 Oct 2019 ∗ This manuscript is based on an article that was published in Brazilian portuguese language in the journal Revista Brasileira de Ensino de Física, 2018, 41, e20180160. http://dx.doi.org/10.1590/ 1806-9126-rbef-2018-0160. -

The R-Process of Stellar Nucleosynthesis: Astrophysics and Nuclear Physics Achievements and Mysteries
The r-process of stellar nucleosynthesis: Astrophysics and nuclear physics achievements and mysteries M. Arnould, S. Goriely, and K. Takahashi Institut d'Astronomie et d'Astrophysique, Universit´eLibre de Bruxelles, CP226, B-1050 Brussels, Belgium Abstract The r-process, or the rapid neutron-capture process, of stellar nucleosynthesis is called for to explain the production of the stable (and some long-lived radioac- tive) neutron-rich nuclides heavier than iron that are observed in stars of various metallicities, as well as in the solar system. A very large amount of nuclear information is necessary in order to model the r-process. This concerns the static characteristics of a large variety of light to heavy nuclei between the valley of stability and the vicinity of the neutron-drip line, as well as their beta-decay branches or their reactivity. Fission probabilities of very neutron-rich actinides have also to be known in order to determine the most massive nuclei that have a chance to be involved in the r-process. Even the properties of asymmetric nuclear matter may enter the problem. The enormously challenging experimental and theoretical task imposed by all these requirements is reviewed, and the state-of-the-art development in the field is presented. Nuclear-physics-based and astrophysics-free r-process models of different levels of sophistication have been constructed over the years. We review their merits and their shortcomings. The ultimate goal of r-process studies is clearly to identify realistic sites for the development of the r-process. Here too, the challenge is enormous, and the solution still eludes us. -

Double-Beta Decay of 96Zr and Double-Electron Capture of 156Dy to Excited Final States
Double-Beta Decay of 96Zr and Double-Electron Capture of 156Dy to Excited Final States by Sean W. Finch Department of Physics Duke University Date: Approved: Werner Tornow, Supervisor Calvin Howell Kate Scholberg Berndt Mueller Albert Chang Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Physics in the Graduate School of Duke University 2015 Abstract Double-Beta Decay of 96Zr and Double-Electron Capture of 156Dy to Excited Final States by Sean W. Finch Department of Physics Duke University Date: Approved: Werner Tornow, Supervisor Calvin Howell Kate Scholberg Berndt Mueller Albert Chang An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Physics in the Graduate School of Duke University 2015 Copyright c 2015 by Sean W. Finch All rights reserved except the rights granted by the Creative Commons Attribution-Noncommercial License Abstract Two separate experimental searches for second-order weak nuclear decays to excited final states were conducted. Both experiments were carried out at the Kimballton Underground Research Facility to provide shielding from cosmic rays. The first search is for the 2νββ decay of 96Zr to excited final states of the daughter nucleus, 96Mo. As a byproduct of this experiment, the β decay of 96Zr was also investigated. Two coaxial high-purity germanium detectors were used in coincidence to detect γ rays produced by the daughter nucleus as it de-excited to the ground state. After collecting 1.92 years of data with 17.91 g of enriched 96Zr, half-life limits at the level of 1020 yr were produced. -

Nuclear Magic Numbers: New Features Far from Stability
Nuclear magic numbers: new features far from stability O. Sorlin1, M.-G. Porquet2 1Grand Acc´el´erateur National d’Ions Lours (GANIL), CEA/DSM - CNRS/IN2P3, B.P. 55027, F-14076 Caen Cedex 5, France 2Centre de Spectrom´etrie Nucl´eaire et de Spectrom´etrie de Masse (CSNSM), CNRS/IN2P3 - Universit´eParis-Sud, Bˆat 104-108, F-91405 Orsay, France May 19, 2008 Abstract The main purpose of the present manuscript is to review the structural evolution along the isotonic and isotopic chains around the ”traditional” magic numbers 8, 20, 28, 50, 82 and 126. The exotic regions of the chart of nuclides have been explored during the three last decades. Then the postulate of permanent magic numbers was definitely abandoned and the reason for these structural mutations has been in turn searched for. General trends in the evolution of shell closures are discussed using complementary experimental information, such as the binding energies of the orbits bounding the shell gaps, the trends of the first collective states of the even-even semi-magic nuclei, and the behavior of certain single-nucleon states. Each section is devoted to a particular magic number. It describes the underlying physics of the shell evolution which is not yet fully understood and indicates future experimental and theoretical challenges. The nuclear mean field embodies various facets of the Nucleon- Nucleon interaction, among which the spin-orbit and tensor terms play decisive roles in the arXiv:0805.2561v1 [nucl-ex] 16 May 2008 shell evolutions. The present review intends to provide experimental constraints to be used for the refinement of theoretical models aiming at a good description of the existing atomic nuclei and at more accurate predictions of hitherto unreachable systems. -

Nuclear Mass and Stability
CHAPTER 3 Nuclear Mass and Stability Contents 3.1. Patterns of nuclear stability 41 3.2. Neutron to proton ratio 43 3.3. Mass defect 45 3.4. Binding energy 47 3.5. Nuclear radius 48 3.6. Semiempirical mass equation 50 3.7. Valley of $-stability 51 3.8. The missing elements: 43Tc and 61Pm 53 3.8.1. Promethium 53 3.8.2. Technetium 54 3.9. Other modes of instability 56 3.10. Exercises 56 3.11. Literature 57 3.1. Patterns of nuclear stability There are approximately 275 different nuclei which have shown no evidence of radioactive decay and, hence, are said to be stable with respect to radioactive decay. When these nuclei are compared for their constituent nucleons, we find that approximately 60% of them have both an even number of protons and an even number of neutrons (even-even nuclei). The remaining 40% are about equally divided between those that have an even number of protons and an odd number of neutrons (even-odd nuclei) and those with an odd number of protons and an even number of neutrons (odd-even nuclei). There are only 5 stable nuclei known which have both 2 6 10 14 an odd number of protons and odd number of neutrons (odd-odd nuclei); 1H, 3Li, 5B, 7N, and 50 23V. It is significant that the first stable odd-odd nuclei are abundant in the very light elements 2 (the low abundance of 1H has a special explanation, see Ch. 17). The last nuclide is found in low isotopic abundance (0.25%) and we cannot be certain that this nuclide is not unstable to radioactive decay with extremely long half-life.