The Nucleus and Nuclear Instability
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shell Structure of Nuclei Far from Stability H. Grawea and M
FR0106040 Gestion 1NI Doc. Enreg. le J./il/ N" TRN A Shell structure of nuclei far from stability H. Grawea and M. Lewitowiczb a Gesellschafit fur Schwerionenforschung mbH, D-64291 Darmstadt, Germany b Grand Accelerateur National d'lons Lourds, BP 55027, F-14076 Caen, France Submitted to the Special Issue of Nuclear Physics A "Research Opportunities with Accelerated Beams of Radioactive Ions" GANIL P 01 03 Shell structure of nuclei far from stability H. Grawea, M. Lewitowiczb * aGesellschaft fur Schwerionenforschung mbH, D- 64291 Darmstadt, Germany bGrand Accelerateur National d'lons Lourds, BP 55027, F-14076 Caen, France The experimental status of shell structure studies in medium-heavy nuclei far off the line of /^-stability is reviewed. Experimental techniques, signatures for shell closure and expectations for future investigations are discussed for the key regions around 48'56Ni, I00Sn for proton rich nuclei and the neutron-rich N=20 isotones, Ca, Ni and Sn isotopes. PACS: 21.10.Dr, 21.10.Pc, 21.60.Cs, 23.40.Hc, 29.30.-h. Keywords: Shell model, shell closure, single particle energies, exotic nuclei. 1. Introduction Doubly magic nuclei in exotic regions of the nuclidic chart are key points to determine shell structure as documented in single particle energies (SPE) and residual interaction between valence nucleons. The parameters of schematic interactions, entering e.g. the numerous versions of Skyrme-Hartree-Fock calculations, are not well determined by the spectroscopy of nuclei close to /^-stability [1]. Relativistic mean field predictions are hampered by the delicate balance of single particle and spin-orbit potentials, which are determined by the small difference and the large sum, respectively, of the contributions from vector and scalar mesons [2]. -

CME Search Before Isobar Collisions and Blind Analysis from STAR
CME Search Before Isobar Collisions and Blind Analysis From STAR Prithwish Tribedy for the STAR collaboration The 36th Winter Workshop on Nuclear Dynamics 1-7th March, 2020, Puerto Vallarta, Mexico In part supported by Introduction Solenoidal Tracker at RHIC (STAR) RHIC has collided multiple ion species; year 2018 was dedicated to search for effects driven by strong electromagnetic fields by STAR Isobars: Ru+Ru, Zr+Zr @ 200GeV (2018) Low energy: Au+Au 27 GeV (2018) Large systems : U+U, Au+Au @ 200 GeV P.Tribedy, WWND 2 The Chiral Magnetic Effect (Cartoon Picture) Quarks Quarks randomly aligned oriented along B antiquarks I II III IV More More right left handed handed quarks quarks J || B J || -B CME converts chiral imbalance to observable electric current P.Tribedy, WWND 2020 3 New Theory Guidance : Complexity Of An Event Magnetic field map Pb+Pb @ 2.76 TeV Axial charge profile b=11.4 fm, Npart=56 Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 uR>uL 0.2 4 uR<uL 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, Tribedy, Phys. Rev. C 92, Based on: Lappi, Schlichting, Phys. Rev. D 97, 011902 (2015) 034034 (2018) Going beyond cartoon picture: 1) Fluctuations dominate e-by-e physics, 2) B-field & domain size of axial-charge change with √s P.Tribedy, WWND 2020 4 New Theory Guidance : Complexity Of An Event Pb+Pb @ 2.76 TeV Magnetic field b=11.4 fm, N Axial charge profile Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 u 0.2 4 u 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, -
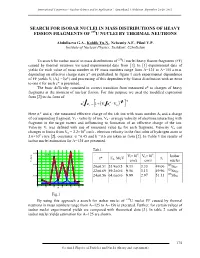
Search for Isobar Nuclei in Mass Distributions of Heavy Fission Fragments of 235U Nuclei by Thermal Neutrons
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 SEARCH FOR ISOBAR NUCLEI IN MASS DISTRIBUTIONS OF HEAVY FISSION FRAGMENTS OF 235U NUCLEI BY THERMAL NEUTRONS Abdullaeva G.A., Koblik Yu.N., Nebesniy A.F., Pikul V.P. Institute of Nuclear Physics, Tashkent, Uzbekistan To search for isobar nuclei in mass distributions of 235U nuclei heavy fission fragments (FF) caused by thermal neutrons we used experimental data from [1]. In [1] experimental data of yields for each value of mass number in FF mass numbers range from A=125 to A=155 a.m.u. depending on effective charge state z* are published. In figure 1 such experimental dependence of FF yields Yi (Ai) =f(z*) and processing of this dependence by Gauss distribution with an error to one σ for each z* is presented. The basic difficulty consisted in correct transition from measured z* to charges of heavy fragments at the moment of nuclear fission. For this purpose we used the modified expression from [2] in the form of: k * α k1 i i ii VzV1zz 0 . Here zi* and zi - the measured effective charge of the i-th ion with mass number Ai and a charge of corresponding fragment, Vi - velocity of ion, Vo - average velocity of electrons interacting with fragment in the target matter and influencing to formation of an effective charge of the ion. Velocity Vi was defined with use of measured value Ek for each fragment. Velocity Vo can 8 changes in limits from Vo = 2.2×10 cm/s - electron velocity in the first orbit of hydrogen atom to 3.6×108 cm/s [2], constants: α =0.45 and k =0.6 are taken as from [2]. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Nuclear Structure Features of Very Heavy and Superheavy Nuclei—Tracing Quantum Mechanics Towards the ‘Island of Stability’
Physica Scripta INVITED COMMENT Related content - Super-heavy nuclei Nuclear structure features of very heavy and Sigurd Hofmann superheavy nuclei—tracing quantum mechanics - New elements - approaching towards the ‘island of stability’ S Hofmann - Review of metastable states in heavy To cite this article: D Ackermann and Ch Theisen 2017 Phys. Scr. 92 083002 nuclei G D Dracoulis, P M Walker and F G Kondev View the article online for updates and enhancements. Recent citations - Production cross section and decay study of Es243 and Md249 R. Briselet et al - Colloquium : Superheavy elements: Oganesson and beyond S. A. Giuliani et al - Neutron stardust and the elements of Earth Brett F. Thornton and Shawn C. Burdette This content was downloaded from IP address 213.131.7.107 on 26/02/2019 at 12:10 | Royal Swedish Academy of Sciences Physica Scripta Phys. Scr. 92 (2017) 083002 (83pp) https://doi.org/10.1088/1402-4896/aa7921 Invited Comment Nuclear structure features of very heavy and superheavy nuclei—tracing quantum mechanics towards the ‘island of stability’ D Ackermann1,2 and Ch Theisen3 1 Grand Accélérateur National d’Ions Lourds—GANIL, CEA/DSM-CNRS/IN2P3, Bd. Becquerel, BP 55027, F-14076 Caen, France 2 GSI Helmholtzzentrum für Schwerionenforschung, Planckstr. 1, D-62491 Darmstadt, Germany 3 Irfu, CEA, Université Paris-Saclay, F-91191 Gif-sur-Yvette, France E-mail: [email protected] Received 13 March 2017, revised 19 May 2017 Accepted for publication 13 June 2017 Published 18 July 2017 Abstract The quantum-mechanic nature of nuclear matter is at the origin of the vision of a region of enhanced stability at the upper right end of the chart of nuclei, the so-called ‘island of stability’. -

Mass Defect & Binding Energy
Sem-3 CC7 Nuclear Physics Mass Defect & Binding Energy According to nuclear particle experiments, the total mass of a nucleus (mnuc) is less than the sum of the masses of its constituent nucleons (protons and neutrons). For a nucleus with Z protons and mass number A, the mass difference or mass defect is given by Δm= Zmp + (A−Z)mn − mnuc where Zmp is the total mass of the protons, (A−Z)mn is the total mass of the neutrons, and mnuc is the mass of the nucleus. According to Einstein’s special theory of relativity, mass is a measure of the total energy of a system (E=mc2). The energy equivalent to mass defect is alled the binding energy B of the nucleus. Thus 2 B= [Zmp + (A−Z)mn − mnuc] c The binding energy is equal to the amount of energy released in forming the nucleus. Example 1. Calculate the mass defect and the binding energy of the deuteron. The mass of the −27 deuteron is mD=3.34359×10 kg or 2.014102u. Solution For the deuteron Z=1 and A=2. The mass defect for the deuteron is Δm=mp+mn−mD=1.008665 u+ 1.007825 u- 2.014102u = 0.002388u The binding energy of the deuteron is then B= Δm c2= Δm ×931.5 MeV/u=2.224MeV. Over two million electron volts are needed to break apart a deuteron into a proton and a neutron. This very large value indicates the great strength of the nuclear force. By comparison, the greatest amount of energy required to liberate an electron bound to a hydrogen atom by an attractive Coulomb force (an electromagnetic force) is about 10 eV. -

Iron-57 of Its Isotopes and Has an Sum of Protons + Neutrons 26 Fe Average Mass As Determined by a in the Nucleus
Elements - elements are pure homogeneous forms of matter Solid Liquid Gas Plamsa •constant volume & shape •constant volume but takes •varaible shape and volume •like a gas except it •very low compressibility on the shape of container that fills the container is composed of ions; •particles vibrate in place •low compressibility •high compressibility an ion is charged •highly ordered arrangement •random particle movement •complete freedom of motion atom or group of •do not flow or diffuse •moderate disorder •extreme disorder atoms. •strongest attractive forces •can flow and diffuse •flow and diffuse easily •examples: between particles •weaker attractive forces •weakest attractive forces - flames •generally more dense than •more dense than gases •least dense state - atmosphere of stars liquids •exert a pressure easily - a comet's tail Matter NO YES Pure Substance Can it be separated Mixture by physical means? Can it be decomposed by Is the mixture composition ordinary chemical means? identical throughout; uniform? NO YES YES NO element compound homogeneous heterogenous mixture mixture Co,Fe,S,H,O,C FeS, H2O, H2SO4 steel, air, blood, steam, wet iron, solution of sulfuric acid Cannot be separated Can be decomposed and rust on steel chemcially into simple chemcially separated Uniform appearance, Appearance, composition, substances into simple substances. composition and and properties are variable properties throughout in different parts of the sample Isotopes Is a mass number attached to the element? NO YES All atoms of the same element are not exactly alike mass number attached mass number equals the 57 The element is as a mixture also can be written as iron-57 of its isotopes and has an sum of protons + neutrons 26 Fe average mass as determined by a in the nucleus. -

Low-Energy Nuclear Physics Part 2: Low-Energy Nuclear Physics
BNL-113453-2017-JA White paper on nuclear astrophysics and low-energy nuclear physics Part 2: Low-energy nuclear physics Mark A. Riley, Charlotte Elster, Joe Carlson, Michael P. Carpenter, Richard Casten, Paul Fallon, Alexandra Gade, Carl Gross, Gaute Hagen, Anna C. Hayes, Douglas W. Higinbotham, Calvin R. Howell, Charles J. Horowitz, Kate L. Jones, Filip G. Kondev, Suzanne Lapi, Augusto Macchiavelli, Elizabeth A. McCutchen, Joe Natowitz, Witold Nazarewicz, Thomas Papenbrock, Sanjay Reddy, Martin J. Savage, Guy Savard, Bradley M. Sherrill, Lee G. Sobotka, Mark A. Stoyer, M. Betty Tsang, Kai Vetter, Ingo Wiedenhoever, Alan H. Wuosmaa, Sherry Yennello Submitted to Progress in Particle and Nuclear Physics January 13, 2017 National Nuclear Data Center Brookhaven National Laboratory U.S. Department of Energy USDOE Office of Science (SC), Nuclear Physics (NP) (SC-26) Notice: This manuscript has been authored by employees of Brookhaven Science Associates, LLC under Contract No.DE-SC0012704 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. -

Range of Usefulness of Bethe's Semiempirical Nuclear Mass Formula
RANGE OF ..USEFULNESS OF BETHE' S SEMIEMPIRIC~L NUCLEAR MASS FORMULA by SEKYU OBH A THESIS submitted to OREGON STATE COLLEGE in partial fulfillment or the requirements tor the degree of MASTER OF SCIENCE June 1956 TTPBOTTDI Redacted for Privacy lrrt rtllrt ?rsfirror of finrrtor Ia Ohrr;r ef lrJer Redacted for Privacy Redacted for Privacy 0hrtrurn of tohoot Om0qat OEt ttm Redacted for Privacy Dru of 0rrdnrtr Sohdbl Drta thrrlr tr prrrEtrl %.ifh , 1,,r, ," r*(,-. ttpo{ by Brtty Drvlr ACKNOWLEDGMENT The author wishes to express his sincere appreciation to Dr. G. L. Trigg for his assistance and encouragement, without which this study would not have been concluded. The author also wishes to thank Dr. E. A. Yunker for making facilities available for these calculations• • TABLE OF CONTENTS Page INTRODUCTION 1 NUCLEAR BINDING ENERGIES AND 5 SEMIEMPIRICAL MASS FORMULA RESEARCH PROCEDURE 11 RESULTS 17 CONCLUSION 21 DATA 29 f BIBLIOGRAPHY 37 RANGE OF USEFULNESS OF BETHE'S SEMIEMPIRICAL NUCLEAR MASS FORMULA INTRODUCTION The complicated experimental results on atomic nuclei haYe been defying definite interpretation of the structure of atomic nuclei for a long tfme. Even though Yarious theoretical methods have been suggested, based upon the particular aspects of experimental results, it has been impossible to find a successful theory which suffices to explain the whole observed properties of atomic nuclei. In 1936, Bohr (J, P• 344) proposed the liquid drop model of atomic nuclei to explain the resonance capture process or nuclear reactions. The experimental evidences which support the liquid drop model are as follows: 1. Substantially constant density of nuclei with radius R - R Al/3 - 0 (1) where A is the mass number of the nucleus and R is the constant of proportionality 0 with the value of (1.5! 0.1) x 10-lJcm~ 2. -

Nuclear Physics
Massachusetts Institute of Technology 22.02 INTRODUCTION to APPLIED NUCLEAR PHYSICS Spring 2012 Prof. Paola Cappellaro Nuclear Science and Engineering Department [This page intentionally blank.] 2 Contents 1 Introduction to Nuclear Physics 5 1.1 Basic Concepts ..................................................... 5 1.1.1 Terminology .................................................. 5 1.1.2 Units, dimensions and physical constants .................................. 6 1.1.3 Nuclear Radius ................................................ 6 1.2 Binding energy and Semi-empirical mass formula .................................. 6 1.2.1 Binding energy ................................................. 6 1.2.2 Semi-empirical mass formula ......................................... 7 1.2.3 Line of Stability in the Chart of nuclides ................................... 9 1.3 Radioactive decay ................................................... 11 1.3.1 Alpha decay ................................................... 11 1.3.2 Beta decay ................................................... 13 1.3.3 Gamma decay ................................................. 15 1.3.4 Spontaneous fission ............................................... 15 1.3.5 Branching Ratios ................................................ 15 2 Introduction to Quantum Mechanics 17 2.1 Laws of Quantum Mechanics ............................................. 17 2.2 States, observables and eigenvalues ......................................... 18 2.2.1 Properties of eigenfunctions ......................................... -

Status of Fission Product Yield Data for Thermal Reactors
Atomic Energy of Canada Limited STATUS OF FISSION PRODUCT YIELD DATA FOR THERMAL REACTORS by W.H. WALKER Chalk River Nuclear Laboratories Chalk River, Ontario February 1974 AECL-4704, tr - STATUS OF FISSION PRODUCT YIELD DATA FOR THERMAL REACTORS W.H. Walker Review Paper lla for the I.A.E.A. Panel on Fission Product Nuclear Data, held at Bologna, Italy, November 26 - 30, 1973. Chalk River Nuclear Laboratories Chalk River, Ontario February 1974 AECL-4704 STATUS OF FISSION PRODUCT YIELD DATA FOR THERMAL REACTORS by W.H. Walker ABSTRACT Methods of measuring fission product yields are discussed. Measured and evaluated chain yields from thermal neutron fission of 233U, 235U, 239Pu and 21flPu are reviewed and evaluated yields are compared in an appendix. Measured fractional and direct yields of interest in reactor cal- culations are discussed and compiled in an appendix. The few data on cumulative yields from fission by low energy epithermal neutrons are noted and their significance in reactor calculations estimated. Chalk River Nuclear Laboratories Chalk River, Ontario February 1974 AECL-4704 Connaissances actuelles, en matière de rendement des produits de fission, pour les réacteurs thermiques par W.H. Walker Résumé Les méthodes permettant de mesurer le rendement des produits de fission font l'objet de commentaires. Des rendements en chaîne ayant été mesurés et évalués à partir 2 3 3 235 239 de la fission, par neutrons thermiques, de U, U, Pu 241 et Pu sont passés en revue et les rendements évalués font l'objet d'une comparaison dans une annexe. Les rende- ments mesurés, directs ou fractionnels, qui présentent un intérêt pour les calculs de réacteur font l'objet de commen- taires et ils sent compilés dans une annexe. -

The Delimiting/Frontier Lines of the Constituents of Matter∗
The delimiting/frontier lines of the constituents of matter∗ Diógenes Galetti1 and Salomon S. Mizrahi2 1Instituto de Física Teórica, Universidade Estadual Paulista (UNESP), São Paulo, SP, Brasily 2Departamento de Física, CCET, Universidade Federal de São Carlos, São Carlos, SP, Brasilz (Dated: October 23, 2019) Abstract Looking at the chart of nuclides displayed at the URL of the International Atomic Energy Agency (IAEA) [1] – that contains all the known nuclides, the natural and those produced artificially in labs – one verifies the existence of two, not quite regular, delimiting lines between which dwell all the nuclides constituting matter. These lines are established by the highly unstable radionuclides located the most far away from those in the central locus, the valley of stability. Here, making use of the “old” semi-empirical mass formula for stable nuclides together with the energy-time uncertainty relation of quantum mechanics, by a simple calculation we show that the obtained frontier lines, for proton and neutron excesses, present an appreciable agreement with the delimiting lines. For the sake of presenting a somewhat comprehensive panorama of the matter in our Universe and their relation with the frontier lines, we narrate, in brief, what is currently known about the astrophysical nucleogenesis processes. Keywords: chart of nuclides, nucleogenesis, nuclide mass formula, valley/line of stability, energy-time un- certainty relation, nuclide delimiting/frontier lines, drip lines arXiv:1909.07223v2 [nucl-th] 22 Oct 2019 ∗ This manuscript is based on an article that was published in Brazilian portuguese language in the journal Revista Brasileira de Ensino de Física, 2018, 41, e20180160. http://dx.doi.org/10.1590/ 1806-9126-rbef-2018-0160.