Radllted by FAST REACTOR NEUTRONS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neutron Interactions and Dosimetry Outline Introduction Tissue
Outline • Neutron dosimetry Neutron Interactions and – Thermal neutrons Dosimetry – Intermediate-energy neutrons – Fast neutrons Chapter 16 • Sources of neutrons • Mixed field dosimetry, paired dosimeters F.A. Attix, Introduction to Radiological • Rem meters Physics and Radiation Dosimetry Introduction Tissue composition • Consider neutron interactions with the majority tissue elements H, O, C, and N, and the resulting absorbed dose • Because of the short ranges of the secondary charged particles that are produced in such interactions, CPE is usually well approximated • Since no bremsstrahlung x-rays are generated, the • The ICRU composition for muscle has been assumed in absorbed dose can be assumed to be equal to the most cases for neutron-dose calculations, lumping the kerma at any point in neutron fields at least up to 1.1% of “other” minor elements together with oxygen to an energy E ~ 20 MeV make a simple four-element (H, O, C, N) composition Neutron kinetic energy Neutron kinetic energy • Neutron fields are divided into three • Thermal neutrons, by definition, have the most probable categories based on their kinetic energy: kinetic energy E=kT=0.025eV at T=20C – Thermal (E<0.5 eV) • Neutrons up to 0.5eV are considered “thermal” due to simplicity of experimental test after they emerge from – Intermediate-energy (0.5 eV<E<10 keV) moderator material – Fast (E>10 keV) • Cadmium ratio test: • Differ by their primary interactions in tissue – Gold foil can be activated through 197Au(n,)198Au interaction and resulting biological effects -

CME Search Before Isobar Collisions and Blind Analysis from STAR
CME Search Before Isobar Collisions and Blind Analysis From STAR Prithwish Tribedy for the STAR collaboration The 36th Winter Workshop on Nuclear Dynamics 1-7th March, 2020, Puerto Vallarta, Mexico In part supported by Introduction Solenoidal Tracker at RHIC (STAR) RHIC has collided multiple ion species; year 2018 was dedicated to search for effects driven by strong electromagnetic fields by STAR Isobars: Ru+Ru, Zr+Zr @ 200GeV (2018) Low energy: Au+Au 27 GeV (2018) Large systems : U+U, Au+Au @ 200 GeV P.Tribedy, WWND 2 The Chiral Magnetic Effect (Cartoon Picture) Quarks Quarks randomly aligned oriented along B antiquarks I II III IV More More right left handed handed quarks quarks J || B J || -B CME converts chiral imbalance to observable electric current P.Tribedy, WWND 2020 3 New Theory Guidance : Complexity Of An Event Magnetic field map Pb+Pb @ 2.76 TeV Axial charge profile b=11.4 fm, Npart=56 Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 uR>uL 0.2 4 uR<uL 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, Tribedy, Phys. Rev. C 92, Based on: Lappi, Schlichting, Phys. Rev. D 97, 011902 (2015) 034034 (2018) Going beyond cartoon picture: 1) Fluctuations dominate e-by-e physics, 2) B-field & domain size of axial-charge change with √s P.Tribedy, WWND 2020 4 New Theory Guidance : Complexity Of An Event Pb+Pb @ 2.76 TeV Magnetic field b=11.4 fm, N Axial charge profile Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 u 0.2 4 u 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, -
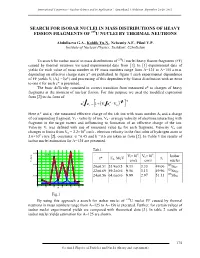
Search for Isobar Nuclei in Mass Distributions of Heavy Fission Fragments of 235U Nuclei by Thermal Neutrons
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 SEARCH FOR ISOBAR NUCLEI IN MASS DISTRIBUTIONS OF HEAVY FISSION FRAGMENTS OF 235U NUCLEI BY THERMAL NEUTRONS Abdullaeva G.A., Koblik Yu.N., Nebesniy A.F., Pikul V.P. Institute of Nuclear Physics, Tashkent, Uzbekistan To search for isobar nuclei in mass distributions of 235U nuclei heavy fission fragments (FF) caused by thermal neutrons we used experimental data from [1]. In [1] experimental data of yields for each value of mass number in FF mass numbers range from A=125 to A=155 a.m.u. depending on effective charge state z* are published. In figure 1 such experimental dependence of FF yields Yi (Ai) =f(z*) and processing of this dependence by Gauss distribution with an error to one σ for each z* is presented. The basic difficulty consisted in correct transition from measured z* to charges of heavy fragments at the moment of nuclear fission. For this purpose we used the modified expression from [2] in the form of: k * α k1 i i ii VzV1zz 0 . Here zi* and zi - the measured effective charge of the i-th ion with mass number Ai and a charge of corresponding fragment, Vi - velocity of ion, Vo - average velocity of electrons interacting with fragment in the target matter and influencing to formation of an effective charge of the ion. Velocity Vi was defined with use of measured value Ek for each fragment. Velocity Vo can 8 changes in limits from Vo = 2.2×10 cm/s - electron velocity in the first orbit of hydrogen atom to 3.6×108 cm/s [2], constants: α =0.45 and k =0.6 are taken as from [2]. -

Compilation and Evaluation of Fission Yield Nuclear Data Iaea, Vienna, 2000 Iaea-Tecdoc-1168 Issn 1011–4289
IAEA-TECDOC-1168 Compilation and evaluation of fission yield nuclear data Final report of a co-ordinated research project 1991–1996 December 2000 The originating Section of this publication in the IAEA was: Nuclear Data Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria COMPILATION AND EVALUATION OF FISSION YIELD NUCLEAR DATA IAEA, VIENNA, 2000 IAEA-TECDOC-1168 ISSN 1011–4289 © IAEA, 2000 Printed by the IAEA in Austria December 2000 FOREWORD Fission product yields are required at several stages of the nuclear fuel cycle and are therefore included in all large international data files for reactor calculations and related applications. Such files are maintained and disseminated by the Nuclear Data Section of the IAEA as a member of an international data centres network. Users of these data are from the fields of reactor design and operation, waste management and nuclear materials safeguards, all of which are essential parts of the IAEA programme. In the 1980s, the number of measured fission yields increased so drastically that the manpower available for evaluating them to meet specific user needs was insufficient. To cope with this task, it was concluded in several meetings on fission product nuclear data, some of them convened by the IAEA, that international co-operation was required, and an IAEA co-ordinated research project (CRP) was recommended. This recommendation was endorsed by the International Nuclear Data Committee, an advisory body for the nuclear data programme of the IAEA. As a consequence, the CRP on the Compilation and Evaluation of Fission Yield Nuclear Data was initiated in 1991, after its scope, objectives and tasks had been defined by a preparatory meeting. -

Wo 2009/108331 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 3 September 2009 (03.09.2009) WO 2009/108331 A2 (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, A61K 38/22 (2006.01) CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (21) International Application Number: HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, PCT/US2009/001213 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (22) International Filing Date: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, 25 February 2009 (25.02.2009) NZ, OM, PG, PH, PL, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, (25) Filing Language: English UG, US, UZ, VC, VN, ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/066,959 25 February 2008 (25.02.2008) US GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, Not furnished 25 February 2009 (25.02.2009) US ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), European (AT, BE, BG, CH, CY, CZ, DE, DK, EE, (71) Applicant and ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (72) Inventor: FORSLEY, Lawrence, Parker, Galloway MC, MK, MT, NL, NO, PL, PT, RO, SE, SI, SK, TR), [US/US]; 70 Elmwood Avenue, Rochester, NY 1461 1 OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, GW, ML, (US). -

Relative Fission Product Yield Determination in the Usgs
RELATIVE FISSION PRODUCT YIELD DETERMINATION IN THE USGS TRIGA MARK I REACTOR by Michael A. Koehl © Copyright by Michael A. Koehl, 2016 All Rights Reserved A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Nuclear Engineering). Golden, Colorado Date: ____________________ Signed: ________________________ Michael A. Koehl Signed: ________________________ Dr. Jenifer C. Braley Thesis Advisor Golden, Colorado Date: ____________________ Signed: ________________________ Dr. Mark P. Jensen Professor and Director Nuclear Science and Engineering Program ii ABSTRACT Fission product yield data sets are one of the most important and fundamental compilations of basic information in the nuclear industry. This data has a wide range of applications which include nuclear fuel burnup and nonproliferation safeguards. Relative fission yields constitute a major fraction of the reported yield data and reduce the number of required absolute measurements. Radiochemical separations of fission products reduce interferences, facilitate the measurement of low level radionuclides, and are instrumental in the analysis of low-yielding symmetrical fission products. It is especially useful in the measurement of the valley nuclides and those on the extreme wings of the mass yield curve, including lanthanides, where absolute yields have high errors. This overall project was conducted in three stages: characterization of the neutron flux in irradiation positions within the U.S. Geological Survey TRIGA Mark I Reactor (GSTR), determining the mass attenuation coefficients of precipitates used in radiochemical separations, and measuring the relative fission products in the GSTR. Using the Westcott convention, the Westcott flux, ; modified spectral index, ; neutron temperature, ; and gold-based cadmium ratiosφ were determined for various sampling√⁄ positions in the USGS TRIGA Mark I reactor. -

Concrete Analysis by Neutron-Capture Gamma Rays Using Californium 252
CONCRETE ANALYSIS BY NEUTRON-CAPTURE GAMMA RAYS USING CALIFORNIUM 252 Dick Duffey, College of Engineering, University of Maryland; Peter F. Wiggins, Naval Systems Engineering Department, U.S. Naval Academy; Frank E. Senftle, U.S. Geological Survey; and A. A. El Kady, United Arab Republic Atomic Energy Establishment, Cairo The feasibility of analyzing concrete and cement by a measurement of the neutron-capture or prompt gamma rays was investigated; a 100-ug californium-252 source was used to supply the neutrons. A lithium drifted germanium crystal detected the capture gamma rays emitted. The capture gamma rays from cement, sand, and 3 coarse aggregates-quartzite gravel, limestone, and diabase--:were studied. Concrete blocks made from these materials were then tested. The capture gamma ray response of the calcium, silicon, and iron in the concrete blocks was in accord with the elements identified in the mix materials. The principal spectral lines used were the 6.42 MeV line of calcium, the 4.93 MeV line of silicon, and the doublet of iron at about 7 .64 MeV. The aluminum line at 7. 72 MeV was ob served in some cases but at a lower intensity with the limited electronic equipment available. This nuclear spectroscopic technique offers a possi ble method of determining the components of sizable concrete samples in a nondestructive, in situ manner. In addition, the neutron-capture gamma ray technique might find application in control of the concrete and cement processes and furnish needed information on production operations and inventories. • FROM THE point of view of the geochemical analyst, concrete may be considered as rock relocated and reformed at the convenience of the engineer. -

The Electronuclear Conversion of Fertile to Fissile Material
UCRL-52144 THE ELECTRONUCLEAR CONVERSION OF FERTILE TO FISSILE MATERIAL C. M. Van Atta J. D. Lee H. Heckrotto October 11, 1976 Prepared for U.S. Energy Research & Development Administration under contract No. W-7405-Eng-48 II\M LAWRENCE lUg LIVERMORE k^tf LABORATORY UnrmsilyotCatftxna/lJvofmofe s$ PC DISTRIBUTION OF THIS DOCUMENmmT IS UMUMWTED NOTICE Thii npoit WM prepared w u account of wot* •pomond by UM Uiilttd Stalwi GovcmiMM. Nittim Uw United Stain nor the United Statn Energy tUwardi it Development AdrnWrtrtUon, not «y of thei* employee!, nor any of their contricton, •ubcontrecton, or their employe*!, makti any warranty, expreai « Implied, or muMi any toga) liability oc reeponafctUty for the accuracy, completenni or uMfulntat of uy Information, apperatui, product or proem dlecloead, or repreienl. that tu UM would not *nfrkig» prrrtt*)}MWiwd r%hl». NOTICE Reference to a oompmy or product rum don not imply approval or nconm ndatjon of the product by the Untnrtity of California or the US. Energy Research A Devetopnient Administration to the uchvton of others that may be suitable. Printed In the United Stitei or America Available from National Technical Information Service U.S. Department of Commerce 528S Port Royal Road Springfield, VA 22161 Price: Printed Copy S : Microfiche $2.25 DMimtic P»t» Ranflt PHca *•#» Rtnft MM 001-025 $ 3.50 326-350 10.00 026-050 4.00 351-375 10.50 051-075 4.50 376-400 10.75 076-100 5.00 401-425 11.00 101-125 5.50 426-450 31.75 126-150 6.00 451-475 12.00 151-175 6.75 476-500 12.50 176-200 7.50 501-525 12.75 201-225 7.75 526-550 13.00 526-250 8.00 551-575 13.50 251-275 9.00 576-600 I3.7S 276-300 9.25 601 -up 301-325 9.75 *Ml J2.50 fot «ch iddltlOMl 100 pip tacmitMt from 601 ID 1,000 flfcK IM 54.50 for eicli iMUknal I0O plfe feenmMI om 1,000 p*o. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

1663-29-Othernuclearreaction.Pdf
it’s not fission or fusion. It’s not alpha, beta, or gamma dosimeter around his neck to track his exposure to radiation decay, nor any other nuclear reaction normally discussed in in the lab. And when he’s not in the lab, he can keep tabs on his an introductory physics textbook. Yet it is responsible for various experiments simultaneously from his office computer the existence of more than two thirds of the elements on the with not one or two but five widescreen monitors—displaying periodic table and is virtually ubiquitous anywhere nuclear graphs and computer codes without a single pixel of unused reactions are taking place—in nuclear reactors, nuclear bombs, space. Data printouts pinned to the wall on the left side of the stellar cores, and supernova explosions. office and techno-scribble densely covering the whiteboard It’s neutron capture, in which a neutron merges with an on the right side testify to a man on a mission: developing, or atomic nucleus. And at first blush, it may even sound deserving at least contributing to, a detailed understanding of complex of its relative obscurity, since neutrons are electrically neutral. atomic nuclei. For that, he’ll need to collect and tabulate a lot For example, add a neutron to carbon’s most common isotope, of cold, hard data. carbon-12, and you just get carbon-13. It’s slightly heavier than Mosby’s primary experimental apparatus for doing this carbon-12, but in terms of how it looks and behaves, the two is the Detector for Advanced Neutron Capture Experiments are essentially identical. -

On the Calculation of the Fast Fission Factor
AE-27 On the Calculation of the w < Fast Fission Factor B. Almgren AKTIEBOLAGET ATOMENERGI STOCKHOLM • SWEDEN • I960 AE-27 ON THE CALCULATION OF THE FAST FISSION FACTOR E. Almgren Summary: Definitions of the fast fission factor e ars discussed. Different methods of calculation of e are compared. Group constants for one -, two- and three- group calculations have been evaluated using the best obtainable basic data. The effects of back-scattering, coupling and (n, 2n)-reactions are discussed. Completion of manuscript in June 1960 Printed and distributed in November 1960 LIST OF CONTENTS Page 1. Definitions 3 2. Formulas for the fast fission factor e and the fast fission ratio R 3 3. Calculation of group constants 7 4. Collision probabilities 10 5. Numerical results 12 6. Discussion 12 7. Acknowledgements 13 Preferences 14 On the calculation of the fast fission factor. 1. Definitions The fast fission factor e may be defined in different ways. Carlvik and Pershagen (3) have defined e as "the number of neutrons which are either slowed down below 0, 1 MeV in the fuel or leave the fuel, per primary neu- tron from thermal fission". This definition has been used in Sweden, since it was proposed (1956). Another commonly used definition is due to Spinrad (2) "the number of neutrons making first collision with moderator per neutron arising from thermal fission". The choice of definition must be consistent with the definition of the resonance escape probability. The above-mentioned definitions include in e some of the capture below the fast fission threshold. However, they do not include the effects of (n, 2n)-reactions or capture of high energy neutrons in the moderator. -

The Nucleus and Nuclear Instability
Lecture 2: The nucleus and nuclear instability Nuclei are described using the following nomenclature: A Z Element N Z is the atomic number, the number of protons: this defines the element. A is called the “mass number” A = N + Z. N is the number of neutrons (N = A - Z) Nuclide: A species of nucleus of a given Z and A. Isotope: Nuclides of an element (i.e. same Z) with different N. Isotone: Nuclides having the same N. Isobar: Nuclides having the same A. [A handy way to keep these straight is to note that isotope includes the letter “p” (same proton number), isotone the letter “n” (same neutron number), and isobar the letter “a” (same A).] Example: 206 82 Pb124 is an isotope of 208 82 Pb126 is an isotone of 207 83 Bi124 and an isobar of 206 84 Po122 1 Chart of the Nuclides Image removed. 90 natural elements 109 total elements All elements with Z > 42 are man-made Except for technicium Z=43 Promethium Z = 61 More than 800 nuclides are known (274 are stable) “stable” unable to transform into another configuration without the addition of outside energy. “unstable” = radioactive Images removed. [www2.bnl.gov/ton] 2 Nuclear Structure: Forces in the nucleus Coulomb Force Force between two point charges, q, separated by distance, r (Coulomb’s Law) k0 q1q2 F(N) = 9 2 -2 r 2 k0 = 8.98755 x 10 N m C (Boltzman constant) Potential energy (MeV) of one particle relative to the other k q q PE(MeV)= 0 1 2 r Strong Nuclear Force • Acts over short distances • ~ 10-15 m • can overcome Coulomb repulsion • acts on protons and neutrons Image removed.