2.) the Liquid Drop Model of the Nucleus Semi-Empirical Mass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CME Search Before Isobar Collisions and Blind Analysis from STAR
CME Search Before Isobar Collisions and Blind Analysis From STAR Prithwish Tribedy for the STAR collaboration The 36th Winter Workshop on Nuclear Dynamics 1-7th March, 2020, Puerto Vallarta, Mexico In part supported by Introduction Solenoidal Tracker at RHIC (STAR) RHIC has collided multiple ion species; year 2018 was dedicated to search for effects driven by strong electromagnetic fields by STAR Isobars: Ru+Ru, Zr+Zr @ 200GeV (2018) Low energy: Au+Au 27 GeV (2018) Large systems : U+U, Au+Au @ 200 GeV P.Tribedy, WWND 2 The Chiral Magnetic Effect (Cartoon Picture) Quarks Quarks randomly aligned oriented along B antiquarks I II III IV More More right left handed handed quarks quarks J || B J || -B CME converts chiral imbalance to observable electric current P.Tribedy, WWND 2020 3 New Theory Guidance : Complexity Of An Event Magnetic field map Pb+Pb @ 2.76 TeV Axial charge profile b=11.4 fm, Npart=56 Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 uR>uL 0.2 4 uR<uL 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, Tribedy, Phys. Rev. C 92, Based on: Lappi, Schlichting, Phys. Rev. D 97, 011902 (2015) 034034 (2018) Going beyond cartoon picture: 1) Fluctuations dominate e-by-e physics, 2) B-field & domain size of axial-charge change with √s P.Tribedy, WWND 2020 4 New Theory Guidance : Complexity Of An Event Pb+Pb @ 2.76 TeV Magnetic field b=11.4 fm, N Axial charge profile Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 u 0.2 4 u 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, -

Probing Pulse Structure at the Spallation Neutron Source Via Polarimetry Measurements
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2017 Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements Connor Miller Gautam University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Nuclear Commons Recommended Citation Gautam, Connor Miller, "Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements. " Master's Thesis, University of Tennessee, 2017. https://trace.tennessee.edu/utk_gradthes/4741 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Connor Miller Gautam entitled "Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Physics. Geoffrey Greene, Major Professor We have read this thesis and recommend its acceptance: Marianne Breinig, Nadia Fomin Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Connor Miller Gautam May 2017 c by Connor Miller Gautam, 2017 All Rights Reserved. -

Nuclear Models: Shell Model
LectureLecture 33 NuclearNuclear models:models: ShellShell modelmodel WS2012/13 : ‚Introduction to Nuclear and Particle Physics ‘, Part I 1 NuclearNuclear modelsmodels Nuclear models Models with strong interaction between Models of non-interacting the nucleons nucleons Liquid drop model Fermi gas model ααα-particle model Optical model Shell model … … Nucleons interact with the nearest Nucleons move freely inside the nucleus: neighbors and practically don‘t move: mean free path λ ~ R A nuclear radius mean free path λ << R A nuclear radius 2 III.III. ShellShell modelmodel 3 ShellShell modelmodel Magic numbers: Nuclides with certain proton and/or neutron numbers are found to be exceptionally stable. These so-called magic numbers are 2, 8, 20, 28, 50, 82, 126 — The doubly magic nuclei: — Nuclei with magic proton or neutron number have an unusually large number of stable or long lived nuclides . — A nucleus with a magic neutron (proton) number requires a lot of energy to separate a neutron (proton) from it. — A nucleus with one more neutron (proton) than a magic number is very easy to separate. — The first exitation level is very high : a lot of energy is needed to excite such nuclei — The doubly magic nuclei have a spherical form Nucleons are arranged into complete shells within the atomic nucleus 4 ExcitationExcitation energyenergy forfor magicm nuclei 5 NuclearNuclear potentialpotential The energy spectrum is defined by the nuclear potential solution of Schrödinger equation for a realistic potential The nuclear force is very short-ranged => the form of the potential follows the density distribution of the nucleons within the nucleus: for very light nuclei (A < 7), the nucleon distribution has Gaussian form (corresponding to a harmonic oscillator potential ) for heavier nuclei it can be parameterised by a Fermi distribution. -
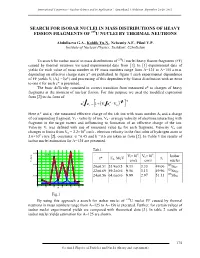
Search for Isobar Nuclei in Mass Distributions of Heavy Fission Fragments of 235U Nuclei by Thermal Neutrons
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 SEARCH FOR ISOBAR NUCLEI IN MASS DISTRIBUTIONS OF HEAVY FISSION FRAGMENTS OF 235U NUCLEI BY THERMAL NEUTRONS Abdullaeva G.A., Koblik Yu.N., Nebesniy A.F., Pikul V.P. Institute of Nuclear Physics, Tashkent, Uzbekistan To search for isobar nuclei in mass distributions of 235U nuclei heavy fission fragments (FF) caused by thermal neutrons we used experimental data from [1]. In [1] experimental data of yields for each value of mass number in FF mass numbers range from A=125 to A=155 a.m.u. depending on effective charge state z* are published. In figure 1 such experimental dependence of FF yields Yi (Ai) =f(z*) and processing of this dependence by Gauss distribution with an error to one σ for each z* is presented. The basic difficulty consisted in correct transition from measured z* to charges of heavy fragments at the moment of nuclear fission. For this purpose we used the modified expression from [2] in the form of: k * α k1 i i ii VzV1zz 0 . Here zi* and zi - the measured effective charge of the i-th ion with mass number Ai and a charge of corresponding fragment, Vi - velocity of ion, Vo - average velocity of electrons interacting with fragment in the target matter and influencing to formation of an effective charge of the ion. Velocity Vi was defined with use of measured value Ek for each fragment. Velocity Vo can 8 changes in limits from Vo = 2.2×10 cm/s - electron velocity in the first orbit of hydrogen atom to 3.6×108 cm/s [2], constants: α =0.45 and k =0.6 are taken as from [2]. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Interaction of Neutrons with Matter
Interaction of Neutrons With Matter § Neutrons interactions depends on energies: from > 100 MeV to < 1 eV § Neutrons are uncharged particles: Þ No interaction with atomic electrons of material Þ interaction with the nuclei of these atoms § The nuclear force, leading to these interactions, is very short ranged Þ neutrons have to pass close to a nucleus to be able to interact ≈ 10-13 cm (nucleus radius) § Because of small size of the nucleus in relation to the atom, neutrons have low probability of interaction Þ long travelling distances in matter See Krane, Segre’, … While bound neutrons in stable nuclei are stable, FREE neutrons are unstable; they undergo beta decay with a lifetime of just under 15 minutes n ® p + e- +n tn = 885.7 ± 0.8 s ≈ 14.76 min Long life times Þ before decaying possibility to interact Þ n physics … x Free neutrons are produced in nuclear fission and fusion x Dedicated neutron sources like research reactors and spallation sources produce free neutrons for the use in irradiation neutron scattering exp. 33 N.B. Vita media del protone: tp > 1.6*10 anni età dell’universo: (13.72 ± 0,12) × 109 anni. beta decay can only occur with bound protons The neutron lifetime puzzle From 2016 Istitut Laue-Langevin (ILL, Grenoble) Annual Report A. Serebrov et al., Phys. Lett. B 605 (2005) 72 A.T. Yue et al., Phys. Rev. Lett. 111 (2013) 222501 Z. Berezhiani and L. Bento, Phys. Rev. Lett. 96 (2006) 081801 G.L. Greene and P. Geltenbort, Sci. Am. 314 (2016) 36 A discrepancy of more than 8 seconds !!!! https://www.scientificamerican.com/article/neutro -

14. Structure of Nuclei Particle and Nuclear Physics
14. Structure of Nuclei Particle and Nuclear Physics Dr. Tina Potter Dr. Tina Potter 14. Structure of Nuclei 1 In this section... Magic Numbers The Nuclear Shell Model Excited States Dr. Tina Potter 14. Structure of Nuclei 2 Magic Numbers Magic Numbers = 2; 8; 20; 28; 50; 82; 126... Nuclei with a magic number of Z and/or N are particularly stable, e.g. Binding energy per nucleon is large for magic numbers Doubly magic nuclei are especially stable. Dr. Tina Potter 14. Structure of Nuclei 3 Magic Numbers Other notable behaviour includes Greater abundance of isotopes and isotones for magic numbers e.g. Z = 20 has6 stable isotopes (average=2) Z = 50 has 10 stable isotopes (average=4) Odd A nuclei have small quadrupole moments when magic First excited states for magic nuclei higher than neighbours Large energy release in α, β decay when the daughter nucleus is magic Spontaneous neutron emitters have N = magic + 1 Nuclear radius shows only small change with Z, N at magic numbers. etc... etc... Dr. Tina Potter 14. Structure of Nuclei 4 Magic Numbers Analogy with atomic behaviour as electron shells fill. Atomic case - reminder Electrons move independently in central potential V (r) ∼ 1=r (Coulomb field of nucleus). Shells filled progressively according to Pauli exclusion principle. Chemical properties of an atom defined by valence (unpaired) electrons. Energy levels can be obtained (to first order) by solving Schr¨odinger equation for central potential. 1 E = n = principle quantum number n n2 Shell closure gives noble gas atoms. Are magic nuclei analogous to the noble gas atoms? Dr. -

1663-29-Othernuclearreaction.Pdf
it’s not fission or fusion. It’s not alpha, beta, or gamma dosimeter around his neck to track his exposure to radiation decay, nor any other nuclear reaction normally discussed in in the lab. And when he’s not in the lab, he can keep tabs on his an introductory physics textbook. Yet it is responsible for various experiments simultaneously from his office computer the existence of more than two thirds of the elements on the with not one or two but five widescreen monitors—displaying periodic table and is virtually ubiquitous anywhere nuclear graphs and computer codes without a single pixel of unused reactions are taking place—in nuclear reactors, nuclear bombs, space. Data printouts pinned to the wall on the left side of the stellar cores, and supernova explosions. office and techno-scribble densely covering the whiteboard It’s neutron capture, in which a neutron merges with an on the right side testify to a man on a mission: developing, or atomic nucleus. And at first blush, it may even sound deserving at least contributing to, a detailed understanding of complex of its relative obscurity, since neutrons are electrically neutral. atomic nuclei. For that, he’ll need to collect and tabulate a lot For example, add a neutron to carbon’s most common isotope, of cold, hard data. carbon-12, and you just get carbon-13. It’s slightly heavier than Mosby’s primary experimental apparatus for doing this carbon-12, but in terms of how it looks and behaves, the two is the Detector for Advanced Neutron Capture Experiments are essentially identical. -

Nuclear Physics: the ISOLDE Facility
Nuclear physics: the ISOLDE facility Lecture 1: Nuclear physics Magdalena Kowalska CERN, EP-Dept. [email protected] on behalf of the CERN ISOLDE team www.cern.ch/isolde Outline Aimed at both physics and non-physics students This lecture: Introduction to nuclear physics Key dates and terms Forces inside atomic nuclei Nuclear landscape Nuclear decay General properties of nuclei Nuclear models Open questions in nuclear physics Lecture 2: CERN-ISOLDE facility Elements of a Radioactive Ion Beam Facility Lecture 3: Physics of ISOLDE Examples of experimental setups and results 2 Small quiz 1 What is Hulk’s connection to the topic of these lectures? Replies should be sent to [email protected] Prize: part of ISOLDE facility 3 Nuclear scale Matter Crystal Atom Atomic nucleus Macroscopic Nucleon Quark Angstrom Nuclear physics: femtometer studies the properties of nuclei and the interactions inside and between them 4 and with a matching theoretical effort theoretical a matching with and facilities experimental dedicated many with better and better it know to getting are we but Today Becquerel, discovery of radioactivity Skłodowska-Curie and Curie, isolation of radium : the exact form of the nuclear interaction is still not known, known, not still is interaction of nuclearthe form exact the : Known nuclides Known Chadwick, neutron discovered History 5 Goeppert-Meyer, Jensen, Haxel, Suess, nuclear shell model first studies on short-lived nuclei Discovery of 1-proton decay Discovery of halo nuclei Discovery of 2-proton decay Calculations with -

The Nucleus and Nuclear Instability
Lecture 2: The nucleus and nuclear instability Nuclei are described using the following nomenclature: A Z Element N Z is the atomic number, the number of protons: this defines the element. A is called the “mass number” A = N + Z. N is the number of neutrons (N = A - Z) Nuclide: A species of nucleus of a given Z and A. Isotope: Nuclides of an element (i.e. same Z) with different N. Isotone: Nuclides having the same N. Isobar: Nuclides having the same A. [A handy way to keep these straight is to note that isotope includes the letter “p” (same proton number), isotone the letter “n” (same neutron number), and isobar the letter “a” (same A).] Example: 206 82 Pb124 is an isotope of 208 82 Pb126 is an isotone of 207 83 Bi124 and an isobar of 206 84 Po122 1 Chart of the Nuclides Image removed. 90 natural elements 109 total elements All elements with Z > 42 are man-made Except for technicium Z=43 Promethium Z = 61 More than 800 nuclides are known (274 are stable) “stable” unable to transform into another configuration without the addition of outside energy. “unstable” = radioactive Images removed. [www2.bnl.gov/ton] 2 Nuclear Structure: Forces in the nucleus Coulomb Force Force between two point charges, q, separated by distance, r (Coulomb’s Law) k0 q1q2 F(N) = 9 2 -2 r 2 k0 = 8.98755 x 10 N m C (Boltzman constant) Potential energy (MeV) of one particle relative to the other k q q PE(MeV)= 0 1 2 r Strong Nuclear Force • Acts over short distances • ~ 10-15 m • can overcome Coulomb repulsion • acts on protons and neutrons Image removed. -

Range of Usefulness of Bethe's Semiempirical Nuclear Mass Formula
RANGE OF ..USEFULNESS OF BETHE' S SEMIEMPIRIC~L NUCLEAR MASS FORMULA by SEKYU OBH A THESIS submitted to OREGON STATE COLLEGE in partial fulfillment or the requirements tor the degree of MASTER OF SCIENCE June 1956 TTPBOTTDI Redacted for Privacy lrrt rtllrt ?rsfirror of finrrtor Ia Ohrr;r ef lrJer Redacted for Privacy Redacted for Privacy 0hrtrurn of tohoot Om0qat OEt ttm Redacted for Privacy Dru of 0rrdnrtr Sohdbl Drta thrrlr tr prrrEtrl %.ifh , 1,,r, ," r*(,-. ttpo{ by Brtty Drvlr ACKNOWLEDGMENT The author wishes to express his sincere appreciation to Dr. G. L. Trigg for his assistance and encouragement, without which this study would not have been concluded. The author also wishes to thank Dr. E. A. Yunker for making facilities available for these calculations• • TABLE OF CONTENTS Page INTRODUCTION 1 NUCLEAR BINDING ENERGIES AND 5 SEMIEMPIRICAL MASS FORMULA RESEARCH PROCEDURE 11 RESULTS 17 CONCLUSION 21 DATA 29 f BIBLIOGRAPHY 37 RANGE OF USEFULNESS OF BETHE'S SEMIEMPIRICAL NUCLEAR MASS FORMULA INTRODUCTION The complicated experimental results on atomic nuclei haYe been defying definite interpretation of the structure of atomic nuclei for a long tfme. Even though Yarious theoretical methods have been suggested, based upon the particular aspects of experimental results, it has been impossible to find a successful theory which suffices to explain the whole observed properties of atomic nuclei. In 1936, Bohr (J, P• 344) proposed the liquid drop model of atomic nuclei to explain the resonance capture process or nuclear reactions. The experimental evidences which support the liquid drop model are as follows: 1. Substantially constant density of nuclei with radius R - R Al/3 - 0 (1) where A is the mass number of the nucleus and R is the constant of proportionality 0 with the value of (1.5! 0.1) x 10-lJcm~ 2. -

Nuclear Physics
Massachusetts Institute of Technology 22.02 INTRODUCTION to APPLIED NUCLEAR PHYSICS Spring 2012 Prof. Paola Cappellaro Nuclear Science and Engineering Department [This page intentionally blank.] 2 Contents 1 Introduction to Nuclear Physics 5 1.1 Basic Concepts ..................................................... 5 1.1.1 Terminology .................................................. 5 1.1.2 Units, dimensions and physical constants .................................. 6 1.1.3 Nuclear Radius ................................................ 6 1.2 Binding energy and Semi-empirical mass formula .................................. 6 1.2.1 Binding energy ................................................. 6 1.2.2 Semi-empirical mass formula ......................................... 7 1.2.3 Line of Stability in the Chart of nuclides ................................... 9 1.3 Radioactive decay ................................................... 11 1.3.1 Alpha decay ................................................... 11 1.3.2 Beta decay ................................................... 13 1.3.3 Gamma decay ................................................. 15 1.3.4 Spontaneous fission ............................................... 15 1.3.5 Branching Ratios ................................................ 15 2 Introduction to Quantum Mechanics 17 2.1 Laws of Quantum Mechanics ............................................. 17 2.2 States, observables and eigenvalues ......................................... 18 2.2.1 Properties of eigenfunctions .........................................