Chapter 2 the Atomic Nucleus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neutron Stars
Chandra X-Ray Observatory X-Ray Astronomy Field Guide Neutron Stars Ordinary matter, or the stuff we and everything around us is made of, consists largely of empty space. Even a rock is mostly empty space. This is because matter is made of atoms. An atom is a cloud of electrons orbiting around a nucleus composed of protons and neutrons. The nucleus contains more than 99.9 percent of the mass of an atom, yet it has a diameter of only 1/100,000 that of the electron cloud. The electrons themselves take up little space, but the pattern of their orbit defines the size of the atom, which is therefore 99.9999999999999% Chandra Image of Vela Pulsar open space! (NASA/PSU/G.Pavlov et al. What we perceive as painfully solid when we bump against a rock is really a hurly-burly of electrons moving through empty space so fast that we can't see—or feel—the emptiness. What would matter look like if it weren't empty, if we could crush the electron cloud down to the size of the nucleus? Suppose we could generate a force strong enough to crush all the emptiness out of a rock roughly the size of a football stadium. The rock would be squeezed down to the size of a grain of sand and would still weigh 4 million tons! Such extreme forces occur in nature when the central part of a massive star collapses to form a neutron star. The atoms are crushed completely, and the electrons are jammed inside the protons to form a star composed almost entirely of neutrons. -

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Arxiv:Nucl-Th/0402046V1 13 Feb 2004
The Shell Model as Unified View of Nuclear Structure E. Caurier,1, ∗ G. Mart´ınez-Pinedo,2,3, † F. Nowacki,1, ‡ A. Poves,4, § and A. P. Zuker1, ¶ 1Institut de Recherches Subatomiques, IN2P3-CNRS, Universit´eLouis Pasteur, F-67037 Strasbourg, France 2Institut d’Estudis Espacials de Catalunya, Edifici Nexus, Gran Capit`a2, E-08034 Barcelona, Spain 3Instituci´oCatalana de Recerca i Estudis Avan¸cats, Llu´ıs Companys 23, E-08010 Barcelona, Spain 4Departamento de F´ısica Te´orica, Universidad Aut´onoma, Cantoblanco, 28049, Madrid, Spain (Dated: October 23, 2018) The last decade has witnessed both quantitative and qualitative progresses in Shell Model stud- ies, which have resulted in remarkable gains in our understanding of the structure of the nucleus. Indeed, it is now possible to diagonalize matrices in determinantal spaces of dimensionality up to 109 using the Lanczos tridiagonal construction, whose formal and numerical aspects we will analyze. Besides, many new approximation methods have been developed in order to overcome the dimensionality limitations. Furthermore, new effective nucleon-nucleon interactions have been constructed that contain both two and three-body contributions. The former are derived from realistic potentials (i.e., consistent with two nucleon data). The latter incorporate the pure monopole terms necessary to correct the bad saturation and shell-formation properties of the real- istic two-body forces. This combination appears to solve a number of hitherto puzzling problems. In the present review we will concentrate on those results which illustrate the global features of the approach: the universality of the effective interaction and the capacity of the Shell Model to describe simultaneously all the manifestations of the nuclear dynamics either of single particle or collective nature. -

Photons from 252Cf and 241Am-Be Neutron Sources H
Neutronenbestrahlungsraum Calibration Laboratory LB6411 Am-Be Quelle [email protected] Photons from 252Cf and 241Am-Be neutron sources H. Hoedlmoser, M. Boschung, K. Meier and S. Mayer Paul Scherrer Institut, CH-5232 Villigen PSI, Switzerland At the accredited PSI calibration laboratory neutron reference fields traceable to the standards of the Physikalisch-Technische Bundesanstalt (PTB) in Germany are available for the calibration of ambient and personal dose equivalent (rate) meters and passive dosimeters. The photon contribution to H*(10) in the neutron fields of the 252Cf and 241Am-Be sources was measured using various photon dose rate meters and active and passive dosimeters. Measuring photons from a neutron source involves considerable uncertainties due to the presence of neutrons, due to a non-zero neutron sensitivity of the photon detector and due to the energy response of the photon detectors. Therefore eight independent detectors and methods were used to obtain a reliable estimate for the photon contribution as an average of the individual methods. For the 241Am-Be source a photon contribution of approximately 4.9% was determined and for the 252Cf source a contribution of 3.6%. 1) Photon detectors 2) Photons from 252Cf and 241Am-Be neutron sources Figure 1 252Cf decays through -emission (~97%) and through spontaneous fission (~3%) with a half-life of 2.65 y. Both processes are accompanied by photon radiation. Furthermore the spectrum of fission products contains radioactive elements that produce additional gamma photons. In the 241Am-Be source 241Am decays through -emission and various low energy emissions: 241Am237Np + + with a half life of 432.6 y. -

Probing Pulse Structure at the Spallation Neutron Source Via Polarimetry Measurements
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2017 Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements Connor Miller Gautam University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Nuclear Commons Recommended Citation Gautam, Connor Miller, "Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements. " Master's Thesis, University of Tennessee, 2017. https://trace.tennessee.edu/utk_gradthes/4741 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Connor Miller Gautam entitled "Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Physics. Geoffrey Greene, Major Professor We have read this thesis and recommend its acceptance: Marianne Breinig, Nadia Fomin Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Probing Pulse Structure at the Spallation Neutron Source via Polarimetry Measurements A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Connor Miller Gautam May 2017 c by Connor Miller Gautam, 2017 All Rights Reserved. -

B2.IV Nuclear and Particle Physics
B2.IV Nuclear and Particle Physics A.J. Barr February 13, 2014 ii Contents 1 Introduction 1 2 Nuclear 3 2.1 Structure of matter and energy scales . 3 2.2 Binding Energy . 4 2.2.1 Semi-empirical mass formula . 4 2.3 Decays and reactions . 8 2.3.1 Alpha Decays . 10 2.3.2 Beta decays . 13 2.4 Nuclear Scattering . 18 2.4.1 Cross sections . 18 2.4.2 Resonances and the Breit-Wigner formula . 19 2.4.3 Nuclear scattering and form factors . 22 2.5 Key points . 24 Appendices 25 2.A Natural units . 25 2.B Tools . 26 2.B.1 Decays and the Fermi Golden Rule . 26 2.B.2 Density of states . 26 2.B.3 Fermi G.R. example . 27 2.B.4 Lifetimes and decays . 27 2.B.5 The flux factor . 28 2.B.6 Luminosity . 28 2.C Shell Model § ............................. 29 2.D Gamma decays § ............................ 29 3 Hadrons 33 3.1 Introduction . 33 3.1.1 Pions . 33 3.1.2 Baryon number conservation . 34 3.1.3 Delta baryons . 35 3.2 Linear Accelerators . 36 iii CONTENTS CONTENTS 3.3 Symmetries . 36 3.3.1 Baryons . 37 3.3.2 Mesons . 37 3.3.3 Quark flow diagrams . 38 3.3.4 Strangeness . 39 3.3.5 Pseudoscalar octet . 40 3.3.6 Baryon octet . 40 3.4 Colour . 41 3.5 Heavier quarks . 43 3.6 Charmonium . 45 3.7 Hadron decays . 47 Appendices 48 3.A Isospin § ................................ 49 3.B Discovery of the Omega § ...................... -

Electron-Nucleon Scattering at LDMX for DUNE
Snowmass Letter of Intent: Snowmass Topical Groups: NF6, RF6, TF11 Electron-Nucleon Scattering at LDMX for DUNE Torsten Akesson1, Artur Ankowski2, Nikita Blinov3, Lene Kristian Bryngemark4, Pierfrancesco Butti2, Caterina Doglioni1, Craig Dukes5, Valentina Dutta6, Bertrand Echenard7, Thomas Eichlersmith8, Ralf Ehrlich5, Andrew Furmanski∗8, Niramay Gogate9, Mathew Graham2, Craig Group5, Alexander Friedland2, David Hitlin7, Vinay Hegde9, Christian Herwig3, Joseph Incandela6, Wesley Ketchumy3, Gordan Krnjaic3, Amina Li6, Shirley Liz2,3, Dexu Lin7, Jeremiah Mans8, Cristina Mantilla Suarez3, Phillip Masterson6, Martin Meier8, Sophie Middleton7, Omar Moreno2, Geoffrey Mullier1, Tim Nelson2, James Oyang7, Gianluca Petrillo2, Ruth Pottgen1, Stefan Prestel1, Luis Sarmiento1, Philip Schuster2, Hirohisa Tanaka2, Lauren Tompkins4, Natalia Toro2, Nhan Tran§3, and Andrew Whitbeck9 1Lund University 2Stanford Linear Accelerator Laboratory 3Fermi National Accelerator Laboratory 4Stanford University 5University of Virginia 6University of California Santa Barbara 7California Institute of Technology 8University of Minnesota 9Texas Tech University ABSTRACT We point out that the LDMX (Light Dark Matter eXperiment) detector design, conceived to search for sub-GeV dark matter, will also have very advantageous characteristics to pursue electron-nucleus scattering measurements of direct relevance to the neutrino program at DUNE and elsewhere. These characteristics include a 4-GeV electron beam, a precision tracker, electromagnetic and hadronic calorimeters with near 2p azimuthal acceptance from the forward beam axis out to 40◦ angle, and low reconstruction energy threshold. LDMX thus could provide (semi)exclusive cross section measurements, with∼ detailed information about final-state electrons, pions, protons, and neutrons. We compare the predictions of two widely used neutrino generators (GENIE, GiBUU) in the LDMX region of acceptance to illustrate the large modeling discrepancies in electron-nucleus interactions at DUNE-like kinematics. -

Nuclear Models: Shell Model
LectureLecture 33 NuclearNuclear models:models: ShellShell modelmodel WS2012/13 : ‚Introduction to Nuclear and Particle Physics ‘, Part I 1 NuclearNuclear modelsmodels Nuclear models Models with strong interaction between Models of non-interacting the nucleons nucleons Liquid drop model Fermi gas model ααα-particle model Optical model Shell model … … Nucleons interact with the nearest Nucleons move freely inside the nucleus: neighbors and practically don‘t move: mean free path λ ~ R A nuclear radius mean free path λ << R A nuclear radius 2 III.III. ShellShell modelmodel 3 ShellShell modelmodel Magic numbers: Nuclides with certain proton and/or neutron numbers are found to be exceptionally stable. These so-called magic numbers are 2, 8, 20, 28, 50, 82, 126 — The doubly magic nuclei: — Nuclei with magic proton or neutron number have an unusually large number of stable or long lived nuclides . — A nucleus with a magic neutron (proton) number requires a lot of energy to separate a neutron (proton) from it. — A nucleus with one more neutron (proton) than a magic number is very easy to separate. — The first exitation level is very high : a lot of energy is needed to excite such nuclei — The doubly magic nuclei have a spherical form Nucleons are arranged into complete shells within the atomic nucleus 4 ExcitationExcitation energyenergy forfor magicm nuclei 5 NuclearNuclear potentialpotential The energy spectrum is defined by the nuclear potential solution of Schrödinger equation for a realistic potential The nuclear force is very short-ranged => the form of the potential follows the density distribution of the nucleons within the nucleus: for very light nuclei (A < 7), the nucleon distribution has Gaussian form (corresponding to a harmonic oscillator potential ) for heavier nuclei it can be parameterised by a Fermi distribution. -

TEK 8.5C: Periodic Table
Name: Teacher: Pd. Date: TEK 8.5C: Periodic Table TEK 8.5C: Interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements. Elements and the Periodic Table An element is a substance that cannot be separated into simpler substances by physical or chemical means. An element is already in its simplest form. The smallest piece of an element that still has the properties of that element is called an atom. An element is a pure substance, containing only one kind of atom. The Periodic Table of Elements is a list of all the elements that have been discovered and named, with each element listed in its own element square. Elements are represented on the Periodic Table by a one or two letter symbol, and its name, atomic number and atomic mass. The Periodic Table & Atomic Structure The elements are listed on the Periodic Table in atomic number order, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s atomic number is based on the number of protons in each atom of that element. In electrically neutral atoms, the atomic number also represents the number of electrons in each atom of that element. For example, the atomic number for neon (Ne) is 10, which means that each atom of neon has 10 protons and 10 electrons. Magnesium (Mg) has an atomic number of 12, which means it has 12 protons and 12 electrons. -

Models of the Atomic Nucleus
Models of the Atomic Nucleus Second Edition Norman D. Cook Models of the Atomic Nucleus Unification Through a Lattice of Nucleons Second Edition 123 Prof. Norman D. Cook Kansai University Dept. Informatics 569 Takatsuki, Osaka Japan [email protected] Additional material to this book can be downloaded from http://extras.springer.com. ISBN 978-3-642-14736-4 e-ISBN 978-3-642-14737-1 DOI 10.1007/978-3-642-14737-1 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2010936431 © Springer-Verlag Berlin Heidelberg 2006, 2010 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Cover design: WMXDesign GmbH, Heidelberg Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) Preface to the Second Edition Already by the 1970s, some theorists had declared that nuclear structure physics was a “closed chapter” in science, but since then it has repeatedly been found necessary to re-open this closed chapter to address old problems and to explain new phenom- ena. -
Pair Energy of Proton and Neutron in Atomic Nuclei
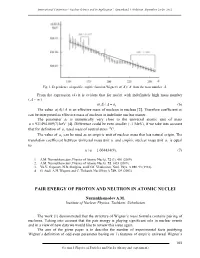
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 Fig. 1. Dependence of specific empiric function Wigner's a()/ A A from the mass number A. From the expression (4) it is evident that for nuclei with indefinitely high mass number ( A ~ ) a()/. A A a1 (6) The value a()/ A A is an effective mass of nucleon in nucleus [2]. Therefore coefficient a1 can be interpreted as effective mass of nucleon in indefinite nuclear matter. The parameter a1 is numerically very close to the universal atomic unit of mass u 931494.009(7) keV [4]. Difference could be even smaller (~1 MeV), if we take into account that for definition of u, used mass of neutral atom 12C. The value of a1 can be used as an empiric unit of nuclear mass that has natural origin. The translation coefficient between universal mass unit u and empiric nuclear mass unit a1 is equal to: u/ a1 1.004434(9). (7) 1. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72 (3), 401 (2009). 2. A.M. Nurmukhamedov, Physics of Atomic Nuclei, 72, 1435 (2009). 3. Yu.V. Gaponov, N.B. Shulgina, and D.M. Vladimirov, Nucl. Phys. A 391, 93 (1982). 4. G. Audi, A.H. Wapstra and C. Thibault, Nucl.Phys A 729, 129 (2003). PAIR ENERGY OF PROTON AND NEUTRON IN ATOMIC NUCLEI Nurmukhamedov A.M. Institute of Nuclear Physics, Tashkent, Uzbekistan The work [1] demonstrated that the structure of Wigner’s mass formula contains pairing of nucleons. Taking into account that the pair energy is playing significant role in nuclear events and in a view of new data we would like to review this issue again. -

From Quark and Nucleon Correlations to Discrete Symmetry and Clustering
From quark and nucleon correlations to discrete symmetry and clustering in nuclei G. Musulmanbekov JINR, Dubna, RU-141980, Russia E-mail: [email protected] Abstract Starting with a quark model of nucleon structure in which the valence quarks are strongly correlated within a nucleon, the light nu- clei are constructed by assuming similar correlations of the quarks of neighboring nucleons. Applying the model to larger collections of nucleons reveals the emergence of the face-centered cubic (FCC) sym- metry at the nuclear level. Nuclei with closed shells possess octahedral symmetry. Binding of nucleons are provided by quark loops formed by three and four nucleon correlations. Quark loops are responsible for formation of exotic (borromean) nuclei, as well. The model unifies independent particle (shell) model, liquid-drop and cluster models. 1 Introduction arXiv:1708.04437v2 [nucl-th] 19 Sep 2017 Historically there are three well known conventional nuclear models based on different assumption about the phase state of the nucleus: the liquid-drop, shell (independent particle), and cluster models. The liquid-drop model re- quires a dense liquid nuclear interior (short mean-free-path, local nucleon interactions and space-occupying nucleons) in order to predict nuclear bind- ing energies, radii, collective oscillations, etc. In contrast, in the shell model each point nucleon moves in mean-field potential created by other nucleons; the model predicts the existence of nucleon orbitals and shell-like orbital- filling. The cluster models require the assumption of strong local-clustering 1 of particularly the 4-nucleon alpha-particle within a liquid or gaseous nuclear interior in order to make predictions about the ground and excited states of cluster configurations.