Stellar Abundances
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CME Search Before Isobar Collisions and Blind Analysis from STAR
CME Search Before Isobar Collisions and Blind Analysis From STAR Prithwish Tribedy for the STAR collaboration The 36th Winter Workshop on Nuclear Dynamics 1-7th March, 2020, Puerto Vallarta, Mexico In part supported by Introduction Solenoidal Tracker at RHIC (STAR) RHIC has collided multiple ion species; year 2018 was dedicated to search for effects driven by strong electromagnetic fields by STAR Isobars: Ru+Ru, Zr+Zr @ 200GeV (2018) Low energy: Au+Au 27 GeV (2018) Large systems : U+U, Au+Au @ 200 GeV P.Tribedy, WWND 2 The Chiral Magnetic Effect (Cartoon Picture) Quarks Quarks randomly aligned oriented along B antiquarks I II III IV More More right left handed handed quarks quarks J || B J || -B CME converts chiral imbalance to observable electric current P.Tribedy, WWND 2020 3 New Theory Guidance : Complexity Of An Event Magnetic field map Pb+Pb @ 2.76 TeV Axial charge profile b=11.4 fm, Npart=56 Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 uR>uL 0.2 4 uR<uL 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, Tribedy, Phys. Rev. C 92, Based on: Lappi, Schlichting, Phys. Rev. D 97, 011902 (2015) 034034 (2018) Going beyond cartoon picture: 1) Fluctuations dominate e-by-e physics, 2) B-field & domain size of axial-charge change with √s P.Tribedy, WWND 2020 4 New Theory Guidance : Complexity Of An Event Pb+Pb @ 2.76 TeV Magnetic field b=11.4 fm, N Axial charge profile Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 u 0.2 4 u 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, -
Search for Isobar Nuclei in Mass Distributions of Heavy Fission Fragments of 235U Nuclei by Thermal Neutrons
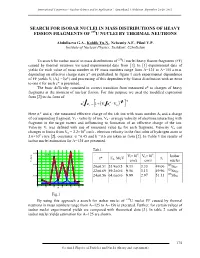
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 SEARCH FOR ISOBAR NUCLEI IN MASS DISTRIBUTIONS OF HEAVY FISSION FRAGMENTS OF 235U NUCLEI BY THERMAL NEUTRONS Abdullaeva G.A., Koblik Yu.N., Nebesniy A.F., Pikul V.P. Institute of Nuclear Physics, Tashkent, Uzbekistan To search for isobar nuclei in mass distributions of 235U nuclei heavy fission fragments (FF) caused by thermal neutrons we used experimental data from [1]. In [1] experimental data of yields for each value of mass number in FF mass numbers range from A=125 to A=155 a.m.u. depending on effective charge state z* are published. In figure 1 such experimental dependence of FF yields Yi (Ai) =f(z*) and processing of this dependence by Gauss distribution with an error to one σ for each z* is presented. The basic difficulty consisted in correct transition from measured z* to charges of heavy fragments at the moment of nuclear fission. For this purpose we used the modified expression from [2] in the form of: k * α k1 i i ii VzV1zz 0 . Here zi* and zi - the measured effective charge of the i-th ion with mass number Ai and a charge of corresponding fragment, Vi - velocity of ion, Vo - average velocity of electrons interacting with fragment in the target matter and influencing to formation of an effective charge of the ion. Velocity Vi was defined with use of measured value Ek for each fragment. Velocity Vo can 8 changes in limits from Vo = 2.2×10 cm/s - electron velocity in the first orbit of hydrogen atom to 3.6×108 cm/s [2], constants: α =0.45 and k =0.6 are taken as from [2]. -

OLLI: the Birth, Life, and Death Of
The Birth, Life, and Death of Stars The Osher Lifelong Learning Institute Florida State University Jorge Piekarewicz Department of Physics [email protected] Schedule: September 29 – November 3 Time: 11:30am – 1:30pm Location: Pepper Center, Broad Auditorium J. Piekarewicz (FSU-Physics) The Birth, Life, and Death of Stars Fall 2014 1 / 12 Ten Compelling Questions What is the raw material for making stars and where did it come from? What forces of nature contribute to energy generation in stars? How and where did the chemical elements form? ? How long do stars live? How will our Sun die? How do massive stars explode? ? What are the remnants of such stellar explosions? What prevents all stars from dying as black holes? What is the minimum mass of a black hole? ? What is role of FSU researchers in answering these questions? J. Piekarewicz (FSU-Physics) The Birth, Life, and Death of Stars Fall 2014 2 / 12 The Birth of Carbon: The Triple-Alpha Reaction The A=5 and A=8 Bottle-Neck 5 −22 p + α ! Li ! p + α (t1=2 ≈10 s) 8 −16 α + α ! Be ! α + α (t1=2 ≈10 s) BBN does not generate any heavy elements! He-ashes fuse in the hot( T ≈108 K) and dense( n≈1028 cm−3) core 8 −8 Physics demands a tiny concentration of Be (n8=n4 ≈10 ) Carbon is formed: α + α ! 8Be + α ! 12C + γ (7:367 MeV) Every atom in our body has been formed in stellar cores! J. Piekarewicz (FSU-Physics) The Birth, Life, and Death of Stars Fall 2014 3 / 12 Stellar Nucleosynthesis: From Carbon to Iron Stars are incredibly efficient thermonuclear furnaces Heavier He-ashes fuse to produce: C,N,O,F,Ne,Na,Mg,.. -

Star Factories: Nuclear Fusion and the Creation of the Elements
Star Factories: Nuclear Fusion and the Creation of the Elements Science In the River City workshop 3/22/11 Chris Taylor Department of Physics and Astronomy Sacramento State Introductions! Science Content Standards, Grades 9 - 12 Earth Sciences: Earth's Place in the Universe 1.e ''Students know the Sun is a typical star and is powered by nuclear reactions, primarily the fusion of hydrogen to form helium.'' 2.c '' Students know the evidence indicating that all elements with an atomic number greater than that of lithium have been formed by nuclear fusion in stars.'' Three topics tonight: 1) how do we know all the heavier elements are made in stars? (Big Bang theory) 2) How do stars make elements as heavy as or less heavy than iron? (Stellar nucleosynthesis) 3) How do stars make elements heavier than iron? (Supernovae) Big Bang Nucleosynthesis The Big Bang theory predicts that when the universe first formed, the only matter that existed was hydrogen, helium, and very tiny amounts of lithium. If this is true, then all other elements must have been created in stars. Astronomers use spectroscopy to examine the light emitted by distant stars to determine what kinds of atoms are in them. We've learned that most stars contain nearly every element in the periodic table. The spectrum of the Sun In order to measure measure what kinds of atoms were around in the earliest days of the Universe, we look for stars that were made out of fresh, primordial gas. The closest we can get to this is looking at dwarf galaxies, which show extremely low levels of elements heavier than helium. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Appendix A: Scientific Notation
Appendix A: Scientific Notation Since in astronomy we often have to deal with large numbers, writing a lot of zeros is not only cumbersome, but also inefficient and difficult to count. Scientists use the system of scientific notation, where the number of zeros is short handed to a superscript. For example, 10 has one zero and is written as 101 in scientific notation. Similarly, 100 is 102, 100 is 103. So we have: 103 equals a thousand, 106 equals a million, 109 is called a billion (U.S. usage), and 1012 a trillion. Now the U.S. federal government budget is in the trillions of dollars, ordinary people really cannot grasp the magnitude of the number. In the metric system, the prefix kilo- stands for 1,000, e.g., a kilogram. For a million, the prefix mega- is used, e.g. megaton (1,000,000 or 106 ton). A billion hertz (a unit of frequency) is gigahertz, although I have not heard of the use of a giga-meter. More rarely still is the use of tera (1012). For small numbers, the practice is similar. 0.1 is 10À1, 0.01 is 10À2, and 0.001 is 10À3. The prefix of milli- refers to 10À3, e.g. as in millimeter, whereas a micro- second is 10À6 ¼ 0.000001 s. It is now trendy to talk about nano-technology, which refers to solid-state device with sizes on the scale of 10À9 m, or about 10 times the size of an atom. With this kind of shorthand convenience, one can really go overboard. -

Nucleosynthesis
Nucleosynthesis Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons, primarily protons and neutrons. The first nuclei were formed about three minutes after the Big Bang, through the process called Big Bang nucleosynthesis. Seventeen minutes later the universe had cooled to a point at which these processes ended, so only the fastest and simplest reactions occurred, leaving our universe containing about 75% hydrogen, 24% helium, and traces of other elements such aslithium and the hydrogen isotope deuterium. The universe still has approximately the same composition today. Heavier nuclei were created from these, by several processes. Stars formed, and began to fuse light elements to heavier ones in their cores, giving off energy in the process, known as stellar nucleosynthesis. Fusion processes create many of the lighter elements up to and including iron and nickel, and these elements are ejected into space (the interstellar medium) when smaller stars shed their outer envelopes and become smaller stars known as white dwarfs. The remains of their ejected mass form theplanetary nebulae observable throughout our galaxy. Supernova nucleosynthesis within exploding stars by fusing carbon and oxygen is responsible for the abundances of elements between magnesium (atomic number 12) and nickel (atomic number 28).[1] Supernova nucleosynthesis is also thought to be responsible for the creation of rarer elements heavier than iron and nickel, in the last few seconds of a type II supernova event. The synthesis of these heavier elements absorbs energy (endothermic process) as they are created, from the energy produced during the supernova explosion. Some of those elements are created from the absorption of multiple neutrons (the r-process) in the period of a few seconds during the explosion. -

Nuclear Astrophysics: the Unfinished Quest for the Origin of the Elements
Nuclear astrophysics: the unfinished quest for the origin of the elements Jordi Jos´e Departament de F´ısica i Enginyeria Nuclear, EUETIB, Universitat Polit`ecnica de Catalunya, E-08036 Barcelona, Spain; Institut d’Estudis Espacials de Catalunya, E-08034 Barcelona, Spain E-mail: [email protected] Christian Iliadis Department of Physics & Astronomy, University of North Carolina, Chapel Hill, North Carolina, 27599, USA; Triangle Universities Nuclear Laboratory, Durham, North Carolina 27708, USA E-mail: [email protected] Abstract. Half a century has passed since the foundation of nuclear astrophysics. Since then, this discipline has reached its maturity. Today, nuclear astrophysics constitutes a multidisciplinary crucible of knowledge that combines the achievements in theoretical astrophysics, observational astronomy, cosmochemistry and nuclear physics. New tools and developments have revolutionized our understanding of the origin of the elements: supercomputers have provided astrophysicists with the required computational capabilities to study the evolution of stars in a multidimensional framework; the emergence of high-energy astrophysics with space-borne observatories has opened new windows to observe the Universe, from a novel panchromatic perspective; cosmochemists have isolated tiny pieces of stardust embedded in primitive meteorites, giving clues on the processes operating in stars as well as on the way matter condenses to form solids; and nuclear physicists have measured reactions near stellar energies, through the combined efforts using stable and radioactive ion beam facilities. This review provides comprehensive insight into the nuclear history of the Universe arXiv:1107.2234v1 [astro-ph.SR] 12 Jul 2011 and related topics: starting from the Big Bang, when the ashes from the primordial explosion were transformed to hydrogen, helium, and few trace elements, to the rich variety of nucleosynthesis mechanisms and sites in the Universe. -

William A. Fowler Papers
http://oac.cdlib.org/findaid/ark:/13030/kt2d5nb7kj No online items Guide to the Papers of William A. Fowler, 1917-1994 Processed by Nurit Lifshitz, assisted by Charlotte Erwin, Laurence Dupray, Carlo Cossu and Jennifer Stine. Archives California Institute of Technology 1200 East California Blvd. Mail Code 015A-74 Pasadena, CA 91125 Phone: (626) 395-2704 Fax: (626) 793-8756 Email: [email protected] URL: http://archives.caltech.edu © 2003 California Institute of Technology. All rights reserved. Guide to the Papers of William A. Consult repository 1 Fowler, 1917-1994 Guide to the Papers of William A. Fowler, 1917-1994 Collection number: Consult repository Archives California Institute of Technology Pasadena, California Contact Information: Archives California Institute of Technology 1200 East California Blvd. Mail Code 015A-74 Pasadena, CA 91125 Phone: (626) 395-2704 Fax: (626) 793-8756 Email: [email protected] URL: http://archives.caltech.edu Processed by: Nurit Lifshitz, assisted by Charlotte Erwin, Laurence Dupray, Carlo Cossu and Jennifer Stine Date Completed: June 2000 Encoded by: Francisco J. Medina. Derived from XML/EAD encoded file by the Center for History of Physics, American Institute of Physics as part of a collaborative project (1999) supported by a grant from the National Endowment for the Humanities. © 2003 California Institute of Technology. All rights reserved. Descriptive Summary Title: William A. Fowler papers, Date (inclusive): 1917-1994 Collection number: Consult repository Creator: Fowler, William A., 1911-1995 Extent: 94 linear feet Repository: California Institute of Technology. Archives. Pasadena, California 91125 Abstract: These papers document the career of William A. Fowler, who served on the physics faculty at California Institute of Technology from 1939 until 1982. -

In-System'' Fission-Events: an Insight Into Puzzles of Exoplanets and Stars?
universe Review “In-System” Fission-Events: An Insight into Puzzles of Exoplanets and Stars? Elizabeth P. Tito 1,* and Vadim I. Pavlov 2,* 1 Scientific Advisory Group, Pasadena, CA 91125, USA 2 Faculté des Sciences et Technologies, Université de Lille, F-59000 Lille, France * Correspondence: [email protected] (E.P.T.); [email protected] (V.I.P.) Abstract: In expansion of our recent proposal that the solar system’s evolution occurred in two stages—during the first stage, the gaseous giants formed (via disk instability), and, during the second stage (caused by an encounter with a particular stellar-object leading to “in-system” fission- driven nucleogenesis), the terrestrial planets formed (via accretion)—we emphasize here that the mechanism of formation of such stellar-objects is generally universal and therefore encounters of such objects with stellar-systems may have occurred elsewhere across galaxies. If so, their aftereffects may perhaps be observed as puzzling features in the spectra of individual stars (such as idiosyncratic chemical enrichments) and/or in the structures of exoplanetary systems (such as unusually high planet densities or short orbital periods). This paper reviews and reinterprets astronomical data within the “fission-events framework”. Classification of stellar systems as “pristine” or “impacted” is offered. Keywords: exoplanets; stellar chemical compositions; nuclear fission; origin and evolution Citation: Tito, E.P.; Pavlov, V.I. “In-System” Fission-Events: An 1. Introduction Insight into Puzzles of Exoplanets As facilities and techniques for astronomical observations and analyses continue to and Stars?. Universe 2021, 7, 118. expand and gain in resolution power, their results provide increasingly detailed information https://doi.org/10.3390/universe about stellar systems, in particular, about the chemical compositions of stellar atmospheres 7050118 and structures of exoplanets. -

The Nucleus and Nuclear Instability
Lecture 2: The nucleus and nuclear instability Nuclei are described using the following nomenclature: A Z Element N Z is the atomic number, the number of protons: this defines the element. A is called the “mass number” A = N + Z. N is the number of neutrons (N = A - Z) Nuclide: A species of nucleus of a given Z and A. Isotope: Nuclides of an element (i.e. same Z) with different N. Isotone: Nuclides having the same N. Isobar: Nuclides having the same A. [A handy way to keep these straight is to note that isotope includes the letter “p” (same proton number), isotone the letter “n” (same neutron number), and isobar the letter “a” (same A).] Example: 206 82 Pb124 is an isotope of 208 82 Pb126 is an isotone of 207 83 Bi124 and an isobar of 206 84 Po122 1 Chart of the Nuclides Image removed. 90 natural elements 109 total elements All elements with Z > 42 are man-made Except for technicium Z=43 Promethium Z = 61 More than 800 nuclides are known (274 are stable) “stable” unable to transform into another configuration without the addition of outside energy. “unstable” = radioactive Images removed. [www2.bnl.gov/ton] 2 Nuclear Structure: Forces in the nucleus Coulomb Force Force between two point charges, q, separated by distance, r (Coulomb’s Law) k0 q1q2 F(N) = 9 2 -2 r 2 k0 = 8.98755 x 10 N m C (Boltzman constant) Potential energy (MeV) of one particle relative to the other k q q PE(MeV)= 0 1 2 r Strong Nuclear Force • Acts over short distances • ~ 10-15 m • can overcome Coulomb repulsion • acts on protons and neutrons Image removed. -

Lecture 7: "Basics of Star Formation and Stellar Nucleosynthesis" Outline
Lecture 7: "Basics of Star Formation and Stellar Nucleosynthesis" Outline 1. Formation of elements in stars 2. Injection of new elements into ISM 3. Phases of star-formation 4. Evolution of stars Mark Whittle University of Virginia Life Cycle of Matter in Milky Way Molecular clouds New clouds with gravitationally collapse heavier composition to form stellar clusters of stars are formed Molecular cloud Stars synthesize Most massive stars evolve He, C, Si, Fe via quickly and die as supernovae – nucleosynthesis heavier elements are injected in space Solar abundances • Observation of atomic absorption lines in the solar spectrum • For some (heavy) elements meteoritic data are used Solar abundance pattern: • Regularities reflect nuclear properties • Several different processes • Mixture of material from many, many stars 5 SolarNucleosynthesis abundances: key facts • Solar• Decreaseabundance in abundance pattern: with atomic number: - Large negative anomaly at Be, B, Li • Regularities reflect nuclear properties - Moderate positive anomaly around Fe • Several different processes 6 - Sawtooth pattern from odd-even effect • Mixture of material from many, many stars Origin of elements • The Big Bang: H, D, 3,4He, Li • All other nuclei were synthesized in stars • Stellar nucleosynthesis ⇔ 3 key processes: - Nuclear fusion: PP cycles, CNO bi-cycle, He burning, C burning, O burning, Si burning ⇒ till 40Ca - Photodisintegration rearrangement: Intense gamma-ray radiation drives nuclear rearrangement ⇒ 56Fe - Most nuclei heavier than 56Fe are due to neutron