NUCLEOSYNTHESIS a Reasonable Starting Point for Isotope Geochemistry Is a Determination of the Abundances of the Naturally Occurring Nuclides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Collapsars As a Major Source of R-Process Elements
Collapsars as a major source of r-process elements Daniel M. Siegel1;2;3;4;5, Jennifer Barnes1;2;5 & Brian D. Metzger1;2 1Department of Physics, Columbia University, New York, NY, USA 2Columbia Astrophysics Laboratory, Columbia University, New York, NY, USA 3Perimeter Institute for Theoretical Physics, Waterloo, Ontario, Canada 4Department of Physics, University of Guelph, Guelph, Ontario, Canada 5NASA Einstein Fellow The production of elements by rapid neutron capture (r-process) in neutron-star mergers is expected theoretically and is supported by multimessenger observations1–3 of gravitational- wave event GW170817: this production route is in principle sufficient to account for most of the r-process elements in the Universe4. Analysis of the kilonova that accompanied GW170817 identified5, 6 delayed outflows from a remnant accretion disk formed around the newly born black hole7–10 as the dominant source of heavy r-process material from that event9, 11. Sim- ilar accretion disks are expected to form in collapsars (the supernova-triggering collapse of rapidly rotating massive stars), which have previously been speculated to produce r-process elements12, 13. Recent observations of stars rich in such elements in the dwarf galaxy Retic- arXiv:1810.00098v2 [astro-ph.HE] 14 Aug 2020 ulum II14, as well as the Galactic chemical enrichment of europium relative to iron over longer timescales15, 16, are more consistent with rare supernovae acting at low stellar metal- licities than with neutron-star mergers. Here we report simulations that show that collapsar accretion disks yield sufficient r-process elements to explain observed abundances in the Uni- 1 verse. Although these supernovae are rarer than neutron- star mergers, the larger amount of material ejected per event compensates for the lower rate of occurrence. -

CME Search Before Isobar Collisions and Blind Analysis from STAR
CME Search Before Isobar Collisions and Blind Analysis From STAR Prithwish Tribedy for the STAR collaboration The 36th Winter Workshop on Nuclear Dynamics 1-7th March, 2020, Puerto Vallarta, Mexico In part supported by Introduction Solenoidal Tracker at RHIC (STAR) RHIC has collided multiple ion species; year 2018 was dedicated to search for effects driven by strong electromagnetic fields by STAR Isobars: Ru+Ru, Zr+Zr @ 200GeV (2018) Low energy: Au+Au 27 GeV (2018) Large systems : U+U, Au+Au @ 200 GeV P.Tribedy, WWND 2 The Chiral Magnetic Effect (Cartoon Picture) Quarks Quarks randomly aligned oriented along B antiquarks I II III IV More More right left handed handed quarks quarks J || B J || -B CME converts chiral imbalance to observable electric current P.Tribedy, WWND 2020 3 New Theory Guidance : Complexity Of An Event Magnetic field map Pb+Pb @ 2.76 TeV Axial charge profile b=11.4 fm, Npart=56 Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 uR>uL 0.2 4 uR<uL 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, Tribedy, Phys. Rev. C 92, Based on: Lappi, Schlichting, Phys. Rev. D 97, 011902 (2015) 034034 (2018) Going beyond cartoon picture: 1) Fluctuations dominate e-by-e physics, 2) B-field & domain size of axial-charge change with √s P.Tribedy, WWND 2020 4 New Theory Guidance : Complexity Of An Event Pb+Pb @ 2.76 TeV Magnetic field b=11.4 fm, N Axial charge profile Pb+Pb 2.76 TeV, b=11.4 fm, dN5/dxT [a.u.] 0.3 6 u 0.2 4 u 0.1 2 0 0 y [fm] -2 -0.1 -4 -0.2 -6 -0.3 -6 -4 -2 0 2 4 6 x [fm] Based on: Chatterjee, -
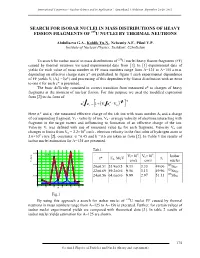
Search for Isobar Nuclei in Mass Distributions of Heavy Fission Fragments of 235U Nuclei by Thermal Neutrons
International Conference “Nuclear Science and its Application”, Samarkand, Uzbekistan, September 25-28, 2012 SEARCH FOR ISOBAR NUCLEI IN MASS DISTRIBUTIONS OF HEAVY FISSION FRAGMENTS OF 235U NUCLEI BY THERMAL NEUTRONS Abdullaeva G.A., Koblik Yu.N., Nebesniy A.F., Pikul V.P. Institute of Nuclear Physics, Tashkent, Uzbekistan To search for isobar nuclei in mass distributions of 235U nuclei heavy fission fragments (FF) caused by thermal neutrons we used experimental data from [1]. In [1] experimental data of yields for each value of mass number in FF mass numbers range from A=125 to A=155 a.m.u. depending on effective charge state z* are published. In figure 1 such experimental dependence of FF yields Yi (Ai) =f(z*) and processing of this dependence by Gauss distribution with an error to one σ for each z* is presented. The basic difficulty consisted in correct transition from measured z* to charges of heavy fragments at the moment of nuclear fission. For this purpose we used the modified expression from [2] in the form of: k * α k1 i i ii VzV1zz 0 . Here zi* and zi - the measured effective charge of the i-th ion with mass number Ai and a charge of corresponding fragment, Vi - velocity of ion, Vo - average velocity of electrons interacting with fragment in the target matter and influencing to formation of an effective charge of the ion. Velocity Vi was defined with use of measured value Ek for each fragment. Velocity Vo can 8 changes in limits from Vo = 2.2×10 cm/s - electron velocity in the first orbit of hydrogen atom to 3.6×108 cm/s [2], constants: α =0.45 and k =0.6 are taken as from [2]. -

The R-Process Nucleosynthesis and Related Challenges
EPJ Web of Conferences 165, 01025 (2017) DOI: 10.1051/epjconf/201716501025 NPA8 2017 The r-process nucleosynthesis and related challenges Stephane Goriely1,, Andreas Bauswein2, Hans-Thomas Janka3, Oliver Just4, and Else Pllumbi3 1Institut d’Astronomie et d’Astrophysique, Université Libre de Bruxelles, CP 226, 1050 Brussels, Belgium 2Heidelberger Institut fr¨ Theoretische Studien, Schloss-Wolfsbrunnenweg 35, 69118 Heidelberg, Germany 3Max-Planck-Institut für Astrophysik, Postfach 1317, 85741 Garching, Germany 4Astrophysical Big Bang Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198, Japan Abstract. The rapid neutron-capture process, or r-process, is known to be of fundamental importance for explaining the origin of approximately half of the A > 60 stable nuclei observed in nature. Recently, special attention has been paid to neutron star (NS) mergers following the confirmation by hydrodynamic simulations that a non-negligible amount of matter can be ejected and by nucleosynthesis calculations combined with the predicted astrophysical event rate that such a site can account for the majority of r-material in our Galaxy. We show here that the combined contribution of both the dynamical (prompt) ejecta expelled during binary NS or NS-black hole (BH) mergers and the neutrino and viscously driven outflows generated during the post-merger remnant evolution of relic BH-torus systems can lead to the production of r-process elements from mass number A > 90 up to actinides. The corresponding abundance distribution is found to reproduce the∼ solar distribution extremely well. It can also account for the elemental distributions observed in low-metallicity stars. However, major uncertainties still affect our under- standing of the composition of the ejected matter. -

Electron Capture in Stars
Electron capture in stars K Langanke1;2, G Mart´ınez-Pinedo1;2;3 and R.G.T. Zegers4;5;6 1GSI Helmholtzzentrum f¨urSchwerionenforschung, D-64291 Darmstadt, Germany 2Institut f¨urKernphysik (Theoriezentrum), Department of Physics, Technische Universit¨atDarmstadt, D-64298 Darmstadt, Germany 3Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨ur Schwerionenforschung, D-64291 Darmstadt, Germany 4 National Superconducting Cyclotron Laboratory, Michigan State University, East Lansing, Michigan 48824, USA 5 Joint Institute for Nuclear Astrophysics: Center for the Evolution of the Elements, Michigan State University, East Lansing, Michigan 48824, USA 6 Department of Physics and Astronomy, Michigan State University, East Lansing, Michigan 48824, USA E-mail: [email protected], [email protected], [email protected] Abstract. Electron captures on nuclei play an essential role for the dynamics of several astrophysical objects, including core-collapse and thermonuclear supernovae, the crust of accreting neutron stars in binary systems and the final core evolution of intermediate mass stars. In these astrophysical objects, the capture occurs at finite temperatures and at densities at which the electrons form a degenerate relativistic electron gas. The capture rates can be derived in perturbation theory where allowed nuclear transitions (Gamow-Teller transitions) dominate, except at the higher temperatures achieved in core-collapse supernovae where also forbidden transitions contribute significantly to the rates. There has been decisive progress in recent years in measuring Gamow-Teller (GT) strength distributions using novel experimental techniques based on charge-exchange reactions. These measurements provide not only data for the GT distributions of ground states for many relevant nuclei, but also serve as valuable constraints for nuclear models which are needed to derive the capture rates for the arXiv:2009.01750v1 [nucl-th] 3 Sep 2020 many nuclei, for which no data exist yet. -

Star Factories: Nuclear Fusion and the Creation of the Elements
Star Factories: Nuclear Fusion and the Creation of the Elements Science In the River City workshop 3/22/11 Chris Taylor Department of Physics and Astronomy Sacramento State Introductions! Science Content Standards, Grades 9 - 12 Earth Sciences: Earth's Place in the Universe 1.e ''Students know the Sun is a typical star and is powered by nuclear reactions, primarily the fusion of hydrogen to form helium.'' 2.c '' Students know the evidence indicating that all elements with an atomic number greater than that of lithium have been formed by nuclear fusion in stars.'' Three topics tonight: 1) how do we know all the heavier elements are made in stars? (Big Bang theory) 2) How do stars make elements as heavy as or less heavy than iron? (Stellar nucleosynthesis) 3) How do stars make elements heavier than iron? (Supernovae) Big Bang Nucleosynthesis The Big Bang theory predicts that when the universe first formed, the only matter that existed was hydrogen, helium, and very tiny amounts of lithium. If this is true, then all other elements must have been created in stars. Astronomers use spectroscopy to examine the light emitted by distant stars to determine what kinds of atoms are in them. We've learned that most stars contain nearly every element in the periodic table. The spectrum of the Sun In order to measure measure what kinds of atoms were around in the earliest days of the Universe, we look for stars that were made out of fresh, primordial gas. The closest we can get to this is looking at dwarf galaxies, which show extremely low levels of elements heavier than helium. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Heavy Element Nucleosynthesis
Heavy Element Nucleosynthesis A summary of the nucleosynthesis of light elements is as follows 4He Hydrogen burning 3He Incomplete PP chain (H burning) 2H, Li, Be, B Non-thermal processes (spallation) 14N, 13C, 15N, 17O CNO processing 12C, 16O Helium burning 18O, 22Ne α captures on 14N (He burning) 20Ne, Na, Mg, Al, 28Si Partly from carbon burning Mg, Al, Si, P, S Partly from oxygen burning Ar, Ca, Ti, Cr, Fe, Ni Partly from silicon burning Isotopes heavier than iron (as well as some intermediate weight iso- topes) are made through neutron captures. Recall that the prob- ability for a non-resonant reaction contained two components: an exponential reflective of the quantum tunneling needed to overcome electrostatic repulsion, and an inverse energy dependence arising from the de Broglie wavelength of the particles. For neutron cap- tures, there is no electrostatic repulsion, and, in complex nuclei, virtually all particle encounters involve resonances. As a result, neutron capture cross-sections are large, and are very nearly inde- pendent of energy. To appreciate how heavy elements can be built up, we must first consider the lifetime of an isotope against neutron capture. If the cross-section for neutron capture is independent of energy, then the lifetime of the species will be ( )1=2 1 1 1 µn τn = ≈ = Nnhσvi NnhσivT Nnhσi 2kT For a typical neutron cross-section of hσi ∼ 10−25 cm2 and a tem- 8 9 perature of 5 × 10 K, τn ∼ 10 =Nn years. Next consider the stability of a neutron rich isotope. If the ratio of of neutrons to protons in an atomic nucleus becomes too large, the nucleus becomes unstable to beta-decay, and a neutron is changed into a proton via − (Z; A+1) −! (Z+1;A+1) + e +ν ¯e (27:1) The timescale for this decay is typically on the order of hours, or ∼ 10−3 years (with a factor of ∼ 103 scatter). -

Minicourses in Astrophysics, Modular Approach, Vol
DOCUMENT RESUME ED 161 706 SE n325 160 ° TITLE Minicourses in Astrophysics, Modular Approach, Vol.. II. INSTITUTION Illinois Univ., Chicago. SPONS AGENCY National Science Foundation, Washington, D. BUREAU NO SED-75-21297 PUB DATE 77 NOTE 134.,; For related document,. see SE 025 159; Contains occasional blurred,-dark print EDRS PRICE MF-$0.83 HC- $7..35 Plus Postage. DESCRIPTORS *Astronomy; *Curriculum Guides; Evolution; Graduate 1:-Uldy; *Higher Edhcation; *InstructionalMaterials; Light; Mathematics; Nuclear Physics; *Physics; Radiation; Relativity: Science Education;. *Short Courses; Space SCiences IDENTIFIERS *Astrophysics , , ABSTRACT . This is the seccA of a two-volume minicourse in astrophysics. It contains chaptez:il on the followingtopics: stellar nuclear energy sources and nucleosynthesis; stellarevolution; stellar structure and its determinatioli;.and pulsars.Each chapter gives much technical discussion, mathematical, treatment;diagrams, and examples. References are-included with each chapter.,(BB) e. **************************************t****************************- Reproductions supplied by EDRS arethe best-that can be made * * . from the original document. _ * .**************************4******************************************** A U S DEPARTMENT OF HEALTH. EDUCATION &WELFARE NATIONAL INSTITUTE OF EDUCATION THIS DOCUMENT AS BEEN REPRO' C'')r.EC! EXACTLY AS RECEIVED FROM THE PERSON OR ORGANIZATION'ORIGIkl A TINC.IT POINTS OF VIEW OR OPINIONS aft STATED DO NOT NECESSARILY REPRE SENT OFFICIAL NATIONAL INSTITUTE Of EDUCATION POSITION OR POLICY s. MINICOURSES IN ASTROPHYSICS MODULAR APPROACH , VOL. II- FA DEVELOPED'AT THE hlIVERSITY OF ILLINOIS AT CHICAGO 1977 I SUPPORTED BY NATIONAL SCIENCE FOUNDATION DIRECTOR: S. SUNDARAM 'ASSOCIATETIRECTOR1 J. BURNS _DEPARTMENT OF PHYSICS. DEPARTMENT OF PHYSICS AND SPACE UNIVERSITY OF ILLINOIS SCIENCES CHICAGO, ILLINOIS 60680 FLORIDA INSTITUTE OF TECHNOLOGY MELBOURNE, FLORIDA 32901 0 0 4, STELLAR NUCLEAR ENERGY SOURCES and NUCLEGSYNTHESIS 'I. -

Sensitivity of Type Ia Supernovae to Electron Capture Rates E
A&A 624, A139 (2019) Astronomy https://doi.org/10.1051/0004-6361/201935095 & c ESO 2019 Astrophysics Sensitivity of Type Ia supernovae to electron capture rates E. Bravo E.T.S. Arquitectura del Vallès, Universitat Politècnica de Catalunya, Carrer Pere Serra 1-15, 08173 Sant Cugat del Vallès, Spain e-mail: [email protected] Received 22 January 2019 / Accepted 8 March 2019 ABSTRACT The thermonuclear explosion of massive white dwarfs is believed to explain at least a fraction of Type Ia supernovae (SNIa). After thermal runaway, electron captures on the ashes left behind by the burning front determine a loss of pressure, which impacts the dynamics of the explosion and the neutron excess of matter. Indeed, overproduction of neutron-rich species such as 54Cr has been deemed a problem of Chandrasekhar-mass models of SNIa for a long time. I present the results of a sensitivity study of SNIa models to the rates of weak interactions, which have been incorporated directly into the hydrodynamic explosion code. The weak rates have been scaled up or down by a factor ten, either globally for a common bibliographical source, or individually for selected isotopes. In line with previous works, the impact of weak rates uncertainties on sub-Chandrasekhar models of SNIa is almost negligible. The impact on the dynamics of Chandrasekhar-mass models and on the yield of 56Ni is also scarce. The strongest effect is found on the nucleosynthesis of neutron-rich nuclei, such as 48Ca, 54Cr, 58Fe, and 64Ni. The species with the highest influence on nucleosynthesis do not coincide with the isotopes that contribute most to the neutronization of matter. -

Nucleosynthesis
Nucleosynthesis Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons, primarily protons and neutrons. The first nuclei were formed about three minutes after the Big Bang, through the process called Big Bang nucleosynthesis. Seventeen minutes later the universe had cooled to a point at which these processes ended, so only the fastest and simplest reactions occurred, leaving our universe containing about 75% hydrogen, 24% helium, and traces of other elements such aslithium and the hydrogen isotope deuterium. The universe still has approximately the same composition today. Heavier nuclei were created from these, by several processes. Stars formed, and began to fuse light elements to heavier ones in their cores, giving off energy in the process, known as stellar nucleosynthesis. Fusion processes create many of the lighter elements up to and including iron and nickel, and these elements are ejected into space (the interstellar medium) when smaller stars shed their outer envelopes and become smaller stars known as white dwarfs. The remains of their ejected mass form theplanetary nebulae observable throughout our galaxy. Supernova nucleosynthesis within exploding stars by fusing carbon and oxygen is responsible for the abundances of elements between magnesium (atomic number 12) and nickel (atomic number 28).[1] Supernova nucleosynthesis is also thought to be responsible for the creation of rarer elements heavier than iron and nickel, in the last few seconds of a type II supernova event. The synthesis of these heavier elements absorbs energy (endothermic process) as they are created, from the energy produced during the supernova explosion. Some of those elements are created from the absorption of multiple neutrons (the r-process) in the period of a few seconds during the explosion. -

The Nucleus and Nuclear Instability
Lecture 2: The nucleus and nuclear instability Nuclei are described using the following nomenclature: A Z Element N Z is the atomic number, the number of protons: this defines the element. A is called the “mass number” A = N + Z. N is the number of neutrons (N = A - Z) Nuclide: A species of nucleus of a given Z and A. Isotope: Nuclides of an element (i.e. same Z) with different N. Isotone: Nuclides having the same N. Isobar: Nuclides having the same A. [A handy way to keep these straight is to note that isotope includes the letter “p” (same proton number), isotone the letter “n” (same neutron number), and isobar the letter “a” (same A).] Example: 206 82 Pb124 is an isotope of 208 82 Pb126 is an isotone of 207 83 Bi124 and an isobar of 206 84 Po122 1 Chart of the Nuclides Image removed. 90 natural elements 109 total elements All elements with Z > 42 are man-made Except for technicium Z=43 Promethium Z = 61 More than 800 nuclides are known (274 are stable) “stable” unable to transform into another configuration without the addition of outside energy. “unstable” = radioactive Images removed. [www2.bnl.gov/ton] 2 Nuclear Structure: Forces in the nucleus Coulomb Force Force between two point charges, q, separated by distance, r (Coulomb’s Law) k0 q1q2 F(N) = 9 2 -2 r 2 k0 = 8.98755 x 10 N m C (Boltzman constant) Potential energy (MeV) of one particle relative to the other k q q PE(MeV)= 0 1 2 r Strong Nuclear Force • Acts over short distances • ~ 10-15 m • can overcome Coulomb repulsion • acts on protons and neutrons Image removed.