Vertical Stratification and Co-Occurrence Patterns of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impact of Imidacloprid and Horticultural Oil on Nonâ•Fitarget
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-2007 Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians Carla Irene Dilling University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Entomology Commons Recommended Citation Dilling, Carla Irene, "Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians. " Master's Thesis, University of Tennessee, 2007. https://trace.tennessee.edu/utk_gradthes/120 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Carla Irene Dilling entitled "Impact of Imidacloprid and Horticultural Oil on Non–target Phytophagous and Transient Canopy Insects Associated with Eastern Hemlock, Tsuga canadensis (L.) Carrieré, in the Southern Appalachians." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Entomology and Plant Pathology. Paris L. Lambdin, Major Professor We have read this thesis and recommend its acceptance: Jerome Grant, Nathan Sanders, James Rhea, Nicole Labbé Accepted for the Council: Carolyn R. -

Arthropods and Other Biota Associated with the Azorean Trees and Shrubs: Juniperus Brevifolia
Arquipelago - Life and Marine Sciences ISSN: 0873-4704 Arthropods and other Biota associated with the Azorean Trees and Shrubs: Juniperus brevifolia RUI NUNES, R. GABRIEL, R.B. ELIAS, F. RIGAL, A.O. SOARES, P. CARDOSO & P.A.V. BORGES Nunes, R., R. Gabriel, R.B. Elias, F. Rigal, A.O. Soares, P. Cardoso & P.A.V. Borges 2015. Arthropods and other Biota associated with the Azorean Trees and Shrubs: Juniperus brevifolia. Arquipelago. Life and Marine Sciences 32: 19-48. Appendix I-IV. This work aims to characterize the arthropods and other biota (lichens, bryophytes, vascular plants and birds) associated with the Azorean endemic tree, Juniperus brevifolia. This is the first of a series of publications that will (i) provide a comprehensive list of all the biota associated with the main Azorean endemic trees and shrubs, (ii) describe in detail the diver- sity, abundance and spatial patterns of canopy arthropods, and (iii) whenever possible, to extend biodiversity assessments to communities of bryophytes, lichens, vascular plants and vertebrates. We use standardized sampled data from BALA project for canopy arthropods and for the remaining taxa we surveyed literature data and the Herbarium of University of Azores. Juniperus brevifolia occurs in a wide range of elevation belts in Azores and accommodates a remarkable large number of taxa: besides canopy arthropods (161 species) it is also an important substrate to other vascular species (six species), bryophytes (105 spe- cies), lichens (106 species) and also birds (four species). In addition, the species richness and particularly the abundance of endemics are dominant, and the number of conservation concern species for bryophytes is noteworthy (30 out of 70). -

Historical Biogeography of Thyrsophorini Psocids and Description of a New Neotropical Species of Thyrsopsocopsis (Psocodea: Psocomorpha: Psocidae)
European Journal of Taxonomy 194: 1–16 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2016.194 www.europeanjournaloftaxonomy.eu 2016 · Román-Palacios C. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:96E9EA43-F6FE-492E-97BE-60DFB8EDE935 Historical biogeography of Thyrsophorini psocids and description of a new neotropical species of Thyrsopsocopsis (Psocodea: Psocomorpha: Psocidae) Cristian ROMÁN-PALACIOS 1,*, Alfonso N. GARCÍA ALDRETE 2 & Ranulfo GONZÁLEZ OBANDO 3 1,3 Departamento de Biología, Facultad de Ciencias Naturales y Exactas, Universidad del Valle, Santiago de Cali, Colombia. 2 Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado Postal 70-153, 04510 Mexico City, Mexico. * Corresponding author: [email protected] 1 urn:lsid:zoobank.org:author:E88D0518-B6CB-4FE7-9EFC-F789EA6F05AD 2 urn:lsid:zoobank.org:author:9E03B921-78AE-4ED6-B1EA-9DCA01BE20BC 3 urn:lsid:zoobank.org:author:16C7AD76-F035-4C8B-8C00-A228CCCD39B0 Abstract. When based on phylogenetic proposals, biogeographic historic narratives have a great interest for hypothesizing paths of origin of the current biodiversity. Among the many questions that remain unsolved about psocids, the distribution of Thyrsophorini represents still a remarkable enigma. This tribe had been considered as exclusively Neotropical, until the description of Thyrsopsocopsis thorntoni Mockford, 2004, from Vietnam. Three hypotheses have been proposed to explain this atypical distribution, recurring to dispersal, vicariance and morphological parallelism between lineages, but the lack of evidence has not allowed a unique support. Here, we describe a new Neotropical species of Thyrsopsocopsis, and also attempt to test the three biogeographical hypotheses in a phylogenetic context. -

BÖCEKLERİN SINIFLANDIRILMASI (Takım Düzeyinde)
BÖCEKLERİN SINIFLANDIRILMASI (TAKIM DÜZEYİNDE) GÖKHAN AYDIN 2016 Editör : Gökhan AYDIN Dizgi : Ziya ÖNCÜ ISBN : 978-605-87432-3-6 Böceklerin Sınıflandırılması isimli eğitim amaçlı hazırlanan bilgisayar programı için lütfen aşağıda verilen linki tıklayarak programı ücretsiz olarak bilgisayarınıza yükleyin. http://atabeymyo.sdu.edu.tr/assets/uploads/sites/76/files/siniflama-05102016.exe Eğitim Amaçlı Bilgisayar Programı ISBN: 978-605-87432-2-9 İçindekiler İçindekiler i Önsöz vi 1. Protura - Coneheads 1 1.1 Özellikleri 1 1.2 Ekonomik Önemi 2 1.3 Bunları Biliyor musunuz? 2 2. Collembola - Springtails 3 2.1 Özellikleri 3 2.2 Ekonomik Önemi 4 2.3 Bunları Biliyor musunuz? 4 3. Thysanura - Silverfish 6 3.1 Özellikleri 6 3.2 Ekonomik Önemi 7 3.3 Bunları Biliyor musunuz? 7 4. Microcoryphia - Bristletails 8 4.1 Özellikleri 8 4.2 Ekonomik Önemi 9 5. Diplura 10 5.1 Özellikleri 10 5.2 Ekonomik Önemi 10 5.3 Bunları Biliyor musunuz? 11 6. Plocoptera – Stoneflies 12 6.1 Özellikleri 12 6.2 Ekonomik Önemi 12 6.3 Bunları Biliyor musunuz? 13 7. Embioptera - webspinners 14 7.1 Özellikleri 15 7.2 Ekonomik Önemi 15 7.3 Bunları Biliyor musunuz? 15 8. Orthoptera–Grasshoppers, Crickets 16 8.1 Özellikleri 16 8.2 Ekonomik Önemi 16 8.3 Bunları Biliyor musunuz? 17 i 9. Phasmida - Walkingsticks 20 9.1 Özellikleri 20 9.2 Ekonomik Önemi 21 9.3 Bunları Biliyor musunuz? 21 10. Dermaptera - Earwigs 23 10.1 Özellikleri 23 10.2 Ekonomik Önemi 24 10.3 Bunları Biliyor musunuz? 24 11. Zoraptera 25 11.1 Özellikleri 25 11.2 Ekonomik Önemi 25 11.3 Bunları Biliyor musunuz? 26 12. -
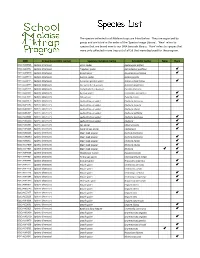
Species List
The species collected in all Malaise traps are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in one trap out of all 59 that were deployed for the program. -

Insecta: Psocodea: 'Psocoptera'
Molecular systematics of the suborder Trogiomorpha (Insecta: Title Psocodea: 'Psocoptera') Author(s) Yoshizawa, Kazunori; Lienhard, Charles; Johnson, Kevin P. Citation Zoological Journal of the Linnean Society, 146(2): 287-299 Issue Date 2006-02 DOI Doc URL http://hdl.handle.net/2115/43134 The definitive version is available at www.blackwell- Right synergy.com Type article (author version) Additional Information File Information 2006zjls-1.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Blackwell Science, LtdOxford, UKZOJZoological Journal of the Linnean Society0024-4082The Lin- nean Society of London, 2006? 2006 146? •••• zoj_207.fm Original Article MOLECULAR SYSTEMATICS OF THE SUBORDER TROGIOMORPHA K. YOSHIZAWA ET AL. Zoological Journal of the Linnean Society, 2006, 146, ••–••. With 3 figures Molecular systematics of the suborder Trogiomorpha (Insecta: Psocodea: ‘Psocoptera’) KAZUNORI YOSHIZAWA1*, CHARLES LIENHARD2 and KEVIN P. JOHNSON3 1Systematic Entomology, Graduate School of Agriculture, Hokkaido University, Sapporo 060-8589, Japan 2Natural History Museum, c.p. 6434, CH-1211, Geneva 6, Switzerland 3Illinois Natural History Survey, 607 East Peabody Drive, Champaign, IL 61820, USA Received March 2005; accepted for publication July 2005 Phylogenetic relationships among extant families in the suborder Trogiomorpha (Insecta: Psocodea: ‘Psocoptera’) 1 were inferred from partial sequences of the nuclear 18S rRNA and Histone 3 and mitochondrial 16S rRNA genes. Analyses of these data produced trees that largely supported the traditional classification; however, monophyly of the infraorder Psocathropetae (= Psyllipsocidae + Prionoglarididae) was not recovered. Instead, the family Psyllipso- cidae was recovered as the sister taxon to the infraorder Atropetae (= Lepidopsocidae + Trogiidae + Psoquillidae), and the Prionoglarididae was recovered as sister to all other families in the suborder. -

Ana Kurbalija PREGLED ENTOMOFAUNE MOČVARNIH
SVEUČILIŠTE JOSIPA JURJA STROSSMAYERA U OSIJEKU I INSTITUT RUĐER BOŠKOVI Ć, ZAGREB Poslijediplomski sveučilišni interdisciplinarni specijalisti čki studij ZAŠTITA PRIRODE I OKOLIŠA Ana Kurbalija PREGLED ENTOMOFAUNE MOČVARNIH STANIŠTA OD MEĐUNARODNOG ZNAČENJA U REPUBLICI HRVATSKOJ Specijalistički rad Osijek, 2012. TEMELJNA DOKUMENTACIJSKA KARTICA Sveučilište Josipa Jurja Strossmayera u Osijeku Specijalistički rad Institit Ruđer Boškovi ć, Zagreb Poslijediplomski sveučilišni interdisciplinarni specijalisti čki studij zaštita prirode i okoliša Znanstveno područje: Prirodne znanosti Znanstveno polje: Biologija PREGLED ENTOMOFAUNE MOČVARNIH STANIŠTA OD ME ĐUNARODNOG ZNAČENJA U REPUBLICI HRVATSKOJ Ana Kurbalija Rad je izrađen na Odjelu za biologiju, Sveučilišta Josipa Jurja Strossmayera u Osijeku Mentor: izv.prof. dr. sc. Stjepan Krčmar U ovom radu je istražen kvalitativni sastav entomof aune na četiri močvarna staništa od me đunarodnog značenja u Republici Hrvatskoj. To su Park prirode Kopački rit, Park prirode Lonjsko polje, Delta rijeke Neretve i Crna Mlaka. Glavni cilj specijalističkog rada je objediniti sve objavljene i neobjavljene podatke o nalazima vrsta kukaca na ova četiri močvarna staništa te kvalitativno usporediti entomofau nu pomoću Sörensonovog indexa faunističke sličnosti. Na području Parka prirode Kopački rit utvrđeno je ukupno 866 vrsta kukaca razvrstanih u 84 porodice i 513 rodova. Na području Parka prirode Lonjsko polje utvrđeno je 513 vrsta kukaca razvrstanih u 24 porodice i 89 rodova. Na području delte rijeke Neretve utvrđeno je ukupno 348 vrsta kukaca razvrstanih u 89 porodica i 227 rodova. Za područje Crne Mlake nije bilo dostupne literature o nalazima kukaca. Velika vrijednost Sörensonovog indexa od 80,85% ukazuje na veliku faunističku sličnost između faune obada Kopačkoga rita i Lonjskoga polja. Najmanja sličnost u fauni obada utvrđena je između močvarnih staništa Lonjskog polja i delte rijeke Neretve, a iznosi 41,37%. -

Psocodea, “Psocoptera”, Psocidae), with One New Species
A peer-reviewed open-access journal ZooKeysA review 203: 27–46 of the(2012) genus Neopsocopsis (Psocodea, “Psocoptera”, Psocidae), with one new species... 27 doi: 10.3897/zookeys.203.3138 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research A review of the genus Neopsocopsis (Psocodea, “Psocoptera”, Psocidae), with one new species from China Lu-Xi Liu1,†, Kazunori Yoshizawa2,‡, Fa-Sheng Li1,§, Zhi-Qi Liu1,| 1 Department of Entomology, China Agricultural University, Beijing, 100193, China 2 Systematic Entomo- logy, Graduate School of Agriculture, Hokkaido University, Sapporo, 060-8589, Japan † urn:lsid:zoobank.org:author:192B5D2C-88C9-41A6-95B5-C6F992B2573B ‡ urn:lsid:zoobank.org:author:E6937129-AF09-4073-BABF-5C025930BF31 § urn:lsid:zoobank.org:author:46BA87D8-F520-4E04-B72A-87901DAFB46E | urn:lsid:zoobank.org:author:A642446F-B2A9-409F-A3D4-0C882890B846 Corresponding author: Zhi-Qi Liu ([email protected]) Academic editor: Vincent Smith | Received 29 March 2012 | Accepted 6 June 2012 | Published 19 June 2012 urn:lsid:zoobank.org:pub:45CC60D2-0723-4177-A271-451D933B8D87 Citation: Liu L-X, Yoshizawa K, Li F-S, Liu Z-Q (2012) A review of the genus Neopsocopsis (Psocodea, “Psocoptera”, Psocidae), with one new species from China. ZooKeys 203: 27–46. doi: 10.3897/zookeys.203.3138 Abstract A review of species of the genus Neopsocopsis Badonnel, 1936 is presented. Four species are redescribed, viz. N. hirticornis (Reuter, 1893), N. quinquedentata (Li & Yang, 1988), N. profunda (Li, 1995), and N. flavida (Li, 1989), as well as the description of one new species, N. convexa sp. n. Seven new synonymies are proposed as follows: Pentablaste obconica Li syn. -

Psocoptera Em Cavernas Do Brasil: Riqueza, Composição E Distribuição
PSOCOPTERA EM CAVERNAS DO BRASIL: RIQUEZA, COMPOSIÇÃO E DISTRIBUIÇÃO THAÍS OLIVEIRA DO CARMO 2009 THAÍS OLIVEIRA DO CARMO PSOCOPTERA EM CAVERNAS DO BRASIL: RIQUEZA, COMPOSIÇÃO E DISTRIBUIÇÃO Dissertação apresentada à Universidade Federal de Lavras, como parte das exigências do programa de Pós-Graduação em Ecologia Aplicada, área de concentração em Ecologia e Conservação de Paisagens Fragmentadas e Agroecossistemas, para obtenção do título de “Mestre”. Orientador Prof. Dr. Rodrigo Lopes Ferreira LAVRAS MINAS GERAIS – BRASIL 2009 Ficha Catalográfica Preparada pela Divisão de Processos Técnicos da Biblioteca Central da UFLA Carmo, Thaís Oliveira do. Psocoptera em cavernas do Brasil: riqueza, composição e distribuição / Thaís Oliveira do Carmo. – Lavras : UFLA, 2009. 98 p. : il. Dissertação (mestrado) – Universidade Federal de Lavras, 2009. Orientador: Rodrigo Lopes Ferreira. Bibliografia. 1. Insetos cavernícolas. 2. Ecologia. 3. Diversidade. 4. Fauna cavernícola. I. Universidade Federal de Lavras. II. Título. CDD – 574.5264 THAÍS OLIVEIRA DO CARMO PSOCOPTERA EM CAVERNAS DO BRASIL: RIQUEZA, COMPOSIÇÃO E DISTRIBUIÇÃO Dissertação apresentada à Universidade Federal de Lavras, como parte das exigências do programa de Pós-Graduação em Ecologia Aplicada, área de concentração em Ecologia e Conservação de Paisagens Fragmentadas e Agroecossistemas, para obtenção do título de “Mestre”. APROVADA em 04 de dezembro de 2009 Prof. Dr. Marconi Souza Silva UNILAVRAS Prof. Dr. Luís Cláudio Paterno Silveira UFLA Prof. Dr. Rodrigo Lopes Ferreira UFLA (Orientador) LAVRAS MINAS GERAIS – BRASIL ...Então não vá embora Agora que eu posso dizer Eu já era o que sou agora Mas agora gosto de ser (Poema Quebrado - Oswaldo Montenegro) AGRADECIMENTOS A Deus, pois com Ele nada nessa vida é impossível! Agradeço aos meus pais, Joaquim e Madalena, pela oportunidade e apoio. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

Psocoptera of Muogamarra Nature Reserve
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Smithers, C. N., 1977. Psocoptera of Muogamarra Nature Reserve. Records of the Australian Museum 31(7): 251–306. [31 December 1977]. doi:10.3853/j.0067-1975.31.1977.215 ISSN 0067-1975 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia THE PSOCOPTERA OF MUOGAMARRA NATURE RESERVE by C. N. SMITHERS The Australian Museum, Sydney SUMMARY In a preliminary study Psocoptera were periodically collected from eight habitats in Muogamarra Nature Reserve, near Sydney. Forty-three species were taken of which fifteen are described as new. Rainforest and Casuarina habitats yielded the greatest variety of species. Populations were highest in summer on Casuarina and showed little seasonal variation in rainforest; dry sclerophyll habitats, on the other hand, yielded greater populations in winter than summer. In each habitat one or two species were dominant. Acacia floribunda and Eucalyptus spp. were poorest in species and specimens. INTRODUCTION During the latter part of 1973 and through most of 1974 collections of Psocoptera were made at intervals from selected habitats in Muogamarra Nature Reserve. Psocoptera had not previously been recorded from the Reserve. The object of the work was to ascertain which species were present and to obtain some indication of any gross habitat preferences or seasonal fluctuations in numbers, if any, as a basis for planning future, more detailed, biological and ecological work. The basic information necessary for rational planning of such work has not been available for Australian species and very little appropriate work which might be applicable to Australian conditions has been done elsewhere. -
Morphology of Psocomorpha (Psocodea: 'Psocoptera')
Title MORPHOLOGY OF PSOCOMORPHA (PSOCODEA: 'PSOCOPTERA') Author(s) Yoshizawa, Kazunori Insecta matsumurana. New series : journal of the Faculty of Agriculture Hokkaido University, series entomology, 62, 1- Citation 44 Issue Date 2005-12 Doc URL http://hdl.handle.net/2115/10524 Type bulletin (article) File Information Yoshizawa-62.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP INSECTA MATSUMURANA NEW SERIES 62: 1–44 DECEMBER 2005 MORPHOLOGY OF PSOCOMORPHA (PSOCODEA: 'PSOCOPTERA') By KAZUNORI YOSHIZAWA Abstract YOSHIZAWA, K. 2005. Morphology of Psocomorpha (Psocodea: 'Psocoptera'). Ins. matsum. n. s. 62: 1–44, 24 figs. Adult integumental morphology of the suborder Psocomorpha (Psocodea: 'Psocoptera') was examined, and homologies and transformation series of characters throughout the suborder and Psocoptera were discussed. These examinations formed the basis of the recent morphology-based cladistic analysis of the Psocomorpha (Yoshizawa, 2002, Zool. J. Linn. Soc. 136: 371–400). Author's address. Systematic Entomology, Graduate School of Agriculture, Hokkaido University, Sapporo, 060-8589 Japan. E-mail. [email protected]. 1 INTRODUCTION Psocoptera (psocids, booklice or barklice) are a paraphyletic assemblage of non-parasitic members of the order Psocodea (Lyal, 1985; Yoshizawa & Johnson, 2003, 2005; Johnson et al., 2004), containing about 5500 described species (Lienhard, 2003). They are about 1 to 10 mm in length and characterized by well-developed postclypeus, long antennae, pick-like lacinia, reduced prothorax, well-developed pterothorax, etc. Phylogenetically, Psocoptera compose a monophyletic group (the order Psocodea) with parasitic lice ('Phtiraptera': biting lice and sucking lice) (Lyal, 1985; Yoshizawa & Johnson, 2003, in press; Johnson et al., 2004). The order is related to Thysanoptera (thrips) and Hemiptera (bugs, cicadas, etc.) (Yoshizawa & Saigusa, 2001, 2003, but see also Yoshizawa & Johnson, 2005).