Arthropods and Other Biota Associated with the Azorean Trees and Shrubs: Juniperus Brevifolia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contribution to the Lepidoptera Fauna of the Madeira Islands Part 2
Beitr. Ent. Keltern ISSN 0005 - 805X 51 (2001) 1 S. 161 - 213 14.09.2001 Contribution to the Lepidoptera fauna of the Madeira Islands Part 2. Tineidae, Acrolepiidae, Epermeniidae With 127 figures Reinhard Gaedike and Ole Karsholt Summary A review of three families Tineidae, Epermeniidae and Acrolepiidae in the Madeira Islands is given. Three new species: Monopis henderickxi sp. n. (Tineidae), Acrolepiopsis mauli sp. n. and A. infundibulosa sp. n. (Acrolepiidae) are described, and two new combinations in the Tineidae: Ceratobia oxymora (MEYRICK) comb. n. and Monopis barbarosi (KOÇAK) comb. n. are listed. Trichophaga robinsoni nom. n. is proposed as a replacement name for the preoccupied T. abrkptella (WOLLASTON, 1858). The first record from Madeira of the family Acrolepiidae (with Acrolepiopsis vesperella (ZELLER) and the two above mentioned new species) is presented, and three species of Tineidae: Stenoptinea yaneimarmorella (MILLIÈRE), Ceratobia oxymora (MEY RICK) and Trichophaga tapetgella (LINNAEUS) are reported as new to the fauna of Madeira. The Madeiran records given for Tsychoidesfilicivora (MEYRICK) are the first records of this species outside the British Isles. Tineapellionella LINNAEUS, Monopis laevigella (DENIS & SCHIFFERMULLER) and M. imella (HÜBNER) are dele ted from the list of Lepidoptera found in Madeira. All species and their genitalia are figured, and informa tion on bionomy is presented. Zusammenfassung Es wird eine Übersicht über die drei Familien Tineidae, Epermeniidae und Acrolepiidae auf den Madeira Inseln gegeben. Die drei neuen Arten Monopis henderickxi sp. n. (Tineidae), Acrolepiopsis mauli sp. n. und A. infundibulosa sp. n. (Acrolepiidae) werden beschrieben, zwei neue Kombinationen bei den Tineidae: Cerato bia oxymora (MEYRICK) comb. -

Molecular Insights Into the Phylogenetic Structure of the Spider
MolecularBlackwell Publishing Ltd insights into the phylogenetic structure of the spider genus Theridion (Araneae, Theridiidae) and the origin of the Hawaiian Theridion-like fauna MIQUEL A. ARNEDO, INGI AGNARSSON & ROSEMARY G. GILLESPIE Accepted: 9 March 2007 Arnedo, M. A., Agnarsson, I. & Gillespie, R. G. (2007). Molecular insights into the phylo- doi:10.1111/j.1463-6409.2007.00280.x genetic structure of the spider genus Theridion (Araneae, Theridiidae) and the origin of the Hawaiian Theridion-like fauna. — Zoologica Scripta, 36, 337–352. The Hawaiian happy face spider (Theridion grallator Simon, 1900), named for a remarkable abdominal colour pattern resembling a smiling face, has served as a model organism for under- standing the generation of genetic diversity. Theridion grallator is one of 11 endemic Hawaiian species of the genus reported to date. Asserting the origin of island endemics informs on the evolutionary context of diversification, and how diversity has arisen on the islands. Studies on the genus Theridion in Hawaii, as elsewhere, have long been hampered by its large size (> 600 species) and poor definition. Here we report results of phylogenetic analyses based on DNA sequences of five genes conducted on five diverse species of Hawaiian Theridion, along with the most intensive sampling of Theridiinae analysed to date. Results indicate that the Hawai- ian Islands were colonised by two independent Theridiinae lineages, one of which originated in the Americas. Both lineages have undergone local diversification in the archipelago and have convergently evolved similar bizarre morphs. Our findings confirm para- or polyphyletic status of the largest Theridiinae genera: Theridion, Achaearanea and Chrysso. -

The Associations Between Pteridophytes and Arthropods
FERN GAZ. 12(1) 1979 29 THE ASSOCIATIONS BETWEEN PTERIDOPHYTES AND ARTHROPODS URI GERSON The Hebrew University of Jerusalem, Faculty of Agriculture, Rehovot, Israel. ABSTRACT Insects belonging to 12 orders, as well as mites, millipedes, woodlice and tardigrades have been collected from Pterldophyta. Primitive and modern, as well as general and specialist arthropods feed on pteridophytes. Insects and mites may cause slight to severe damage, all plant parts being susceptible. Several arthropods are pests of commercial Pteridophyta, their control being difficult due to the plants' sensitivity to pesticides. Efforts are currently underway to employ insects for the biological control of bracken and water ferns. Although Pteridophyta are believed to be relatively resistant to arthropods, the evidence is inconclusive; pteridophyte phytoecdysones do not appear to inhibit insect feeders. Other secondary compounds of preridophytes, like prunasine, may have a more important role in protecting bracken from herbivores. Several chemicals capable of adversely affecting insects have been extracted from Pteridophyta. The litter of pteridophytes provides a humid habitat for various parasitic arthropods, like the sheep tick. Ants often abound on pteridophytes (especially in the tropics) and may help in protecting these plants while nesting therein. These and other associations are discussed . lt is tenatively suggested that there might be a difference in the spectrum of arthropods attacking ancient as compared to modern Pteridophyta. The Osmundales, which, in contrast to other ancient pteridophytes, contain large amounts of ·phytoecdysones, are more similar to modern Pteridophyta in regard to their arthropod associates. The need for further comparative studies is advocated, with special emphasis on the tropics. -

Phylogenetic Analyses of Juniperus Species in Turkey and Their Relations with Other Juniperus Based on Cpdna Supervisor: Prof
MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY AYSUN DEMET GÜVENDİREN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY APRIL 2015 Approval of the thesis MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA submitted by AYSUN DEMET GÜVENDİREN in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Department of Biological Sciences, Middle East Technical University by, Prof. Dr. Gülbin Dural Ünver Dean, Graduate School of Natural and Applied Sciences Prof. Dr. Orhan Adalı Head of the Department, Biological Sciences Prof. Dr. Zeki Kaya Supervisor, Dept. of Biological Sciences METU Examining Committee Members Prof. Dr. Musa Doğan Dept. Biological Sciences, METU Prof. Dr. Zeki Kaya Dept. Biological Sciences, METU Prof.Dr. Hayri Duman Biology Dept., Gazi University Prof. Dr. İrfan Kandemir Biology Dept., Ankara University Assoc. Prof. Dr. Sertaç Önde Dept. Biological Sciences, METU Date: iii I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name : Aysun Demet GÜVENDİREN Signature : iv ABSTRACT MOLECULAR PHYLOGENETIC ANALYSES OF JUNIPERUS L. SPECIES IN TURKEY AND THEIR RELATIONS WITH OTHER JUNIPERS BASED ON cpDNA Güvendiren, Aysun Demet Ph.D., Department of Biological Sciences Supervisor: Prof. -

Parasites (Hymenoptera: Braconidae) Near Belleville, Ontario, Canada
Naturaliste can - 1 07: 87-93 (1980). PLANT BUG HOSTS (HETEROPTERA: MIRIDAE) OF SOME EUPHORINE PARASITES (HYMENOPTERA: BRACONIDAE) NEAR BELLEVILLE, ONTARIO, CANADA C. C. LOAN Biosystematics Research Institute, Agriculture Canada, Research Branch, Ottawa KlA 0C6 Resume Nous avons e'eve des Euphorines parasites (14 espbces de Peristenus et 4 de Leiophron) b partir de 28 especes de Mirides, recoltes pres de Belleville, Ontario. Nous avons, en plus, obtenu plusieurs immatures d'Euphorines indeterminees chez 24 autres espbces d'hotes. Les parasites de chaque espece se rencontrent dans les nymphes d'une ou plusieurs especes de mirides. La majorite des hates et tous les parasites nont qu'une seule generation annuelle. L'attaque des para- sites ne se produit que durant la periode nymphale de I'hote. Les adultes hivernent en diapause, dans les cocons. Le taux de parasitisme est de 16 6 64%. Abstract Euphorine parasites, comprising 14 species of Peristenus and four of Leio- phron, were reared from 28 plant bug species collected near Belleville, Ontario. Immature, unidentifiable euphorines were found in 24 other host species. Each of the parasite species attacked nymphs of one or more plant bugs. Most of the hosts, and all the parasites have one generation per year. Parasitism was limited to the portion of the season when the host(s) was in the nymphal stage. The over- wintered parasites were inactive as diapausing adults in cocoons until the growing season of the following year. From 16-64 per cent of host nymphs were parasitized. Introduction Materials and methods Species of the euphorine genera Periste- Plant bugs were collected during May- nus Foerster and Leiophron Nees parasitize August in representative habitats immediately nymphs of plant bugs (Miridae). -
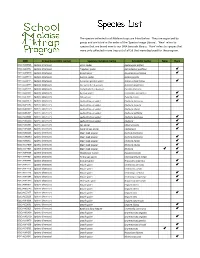
Species List
The species collected in all Malaise traps are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in one trap out of all 59 that were deployed for the program. -

Potential Natural Vegetation and Pre-Anthropic Pollen Records on the Azores
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Sapientia 1 1 Correspondence 2 2485 words 3 4 5 6 Potential natural vegetation and pre-anthropic pollen records on the Azores 7 Islands in a Macaronesian context 8 9 10 Valentí Rull1*, Simon E. Connor2,3 & Rui B. Elias4 11 12 13 1Institute of Earth Sciences Jaume Almera (ICTJA-CSIC), 08028 Barcelona, Spain 14 2School of Geography, University of Melbourne, VIC-3010, Australia 15 3CIMA-FCT, Universidade do Algarve, Faro 8005-139, Portugal 16 4Centre for Ecology, Evolution and Environmental Change (CE3C), Universidade dos Açores, Angra do 17 Heroísmo (Açores), Portugal 18 19 20 *Corresponding author: Email [email protected] 21 22 23 24 25 2 26 Abstract 27 28 This paper discusses the concept of potential natural vegetation (PNV) in light of the pollen records 29 available to date for the Macaronesian biogeographical region, with emphasis on the Azores Islands. The 30 classical debate on the convenience or not of the PNV concept has been recently revived in the Canary 31 Islands, where pollen records of pre-anthropic vegetation seemed to strongly disagree with the existing 32 PNV reconstructions. Contrastingly, more recent PNV model outputs from the Azores Islands show 33 outstanding parallelisms with pre-anthropic pollen records, at least in qualitative terms. We suggest the 34 development of more detailed quantitative studies to compare these methodologies as an opportunity 35 for improving the performance of both. PNV modelling may benefit by incorporating empirical data on 36 past vegetation useful for calibration and validation purposes, whereas palynology may improve past 37 reconstructions by minimizing interpretative biases linked to differential pollen production, dispersal 38 and preservation. -

Stenophylla Lobivertex Lombardo, Nuevo Registro De Mántido Para La Fauna Colombiana (Insecta: Mantodea)*
STENOPHYLLA LOBIVERTEX LOMBARDO, NUEVO REGISTRO DE MÁNTIDO PARA LA FAUNA COLOMBIANA (INSECTA: MANTODEA)* Julián A. Salazar-E.1 Resumen Se registra por primera vez para Colombia, la especie Stenophylla lobivertex (LOMBARDO, 2000), mediante un macho depositado en la colección del autor, Manizales. Palabras clave Colombia, Mantodea, Acontistinae, Stenophylla lobivertex, Putumayo, registro. STENOPHYLLA LOBIVERTEX LOMBARDO, A NEW RECORD FOR COLOMBIAN FAUNA (INSECTA: MANTODEA) Abstract The Stenophylla lobivertex (Lombardo, 2000) species is recorded for the first time in Colombia, through a male deposited in the author’s collection, in Manizales. Key words Colombia, Mantodea, Acontistinae, Stenophylla lobivertex, Putumayo, record. INTRODUCCIÓN El Neotrópico es la tercera región en el mundo más rica en especies de Mantodea (WERNER, 1909; OTTE & SPEARMAN, 2005). Por esto, permanentemente hay sorpresas cada vez que salimos al campo a buscarlas. Siguiendo los registros de mantis para Colombia previamente descritos en países vecinos, y que han sido publicados en esta revista por SALAZAR (2000, 2001, 2002a, 2003) y otros autores (VELÁSQUEZ-TIBATÁ, 2000; AGUDELO & CHICA, 2001), hacemos esta nota para anunciar el descubrimiento en nuestro país de S. lobivertex, una extraordinaria especie de reciente descripción para el área Amazónica de Ecuador y * Recibido 13 de marzo de 2007, aceptado 18 de abril de 2007. 1 M. V. S., Centro de Museos, Historia Natural, Universidad de Caldas, A.A. 275, Manizales, Colombia. Stenophylla Lobivertex lombardo, nuevo registro de mántido para la fauna colombiana (insecta: mantodea) Perú. Lo anterior permite adicionar el género Stenophylla Ww.,1843 a la mantidofauna colombiana con una lista publicada previamente por el presente autor hace cinco años (SALAZAR, 2002b). -

The Subterranean Fauna of a Biodiversity Hotspot Region-Portugal: an Overview and Its Conservation
International Journal of Speleology 40 (1) 23-37 Tampa, FL (USA) January 2011 Available online at www.ijs.speleo.it & scholarcommons.usf.edu/ijs/ International Journal of Speleology Official Journal of Union Internationale de Spéléologie The subterranean fauna of a biodiversity hotspot region - Portugal: an overview and its conservation Ana Sofia P.S. Reboleira1, 2, *, Paulo A.V. Borges3, Fernando Gonçalves1, Artur R.M. Serrano4 & Pedro Oromí2 Abstract: Reboleira A.S.P.S., Borges P.A.V., Gonçalves F., Serrano A.R.M., Oromi P. 2011. The subterranean fauna of a biodiversity hotspot region - Portugal: an overview and its conservation. International Journal of Speleology, 40 (1), 23-37. Tampa, FL (USA). ISSN 0392-6672. DOI: 10.5038/1827-806X.40.1.4 An overview of the obligate hypogean fauna in Portugal (including Azores and Madeira archipelagos) is provided, with a list of obligated cave-dwelling species and subspecies, and a general perspective about its conservation. All the available literature on subterranean Biology of Portugal since the first written record in 1870 until today has been revised. A total of 43 troglobiont and 67 stygobiont species and subspecies from 12 orders have been described so far in these areas, included in the so-called Mediterranean hotspot of biodiversity. The subterranean fauna in Portugal has been considered moderately poor with some endemic relicts and it remains to be demonstrated if this fact is still true after investing in standard surveys in cave environments. The major problems related to the conservation of cave fauna are discussed, but it is clear that the protection of this specialized fauna implies an adequate management of surface habitats. -
![BIONOZOS of SOIL] STM4T2STING AOULIM HILLOOPTERA. by HUGH](https://docslib.b-cdn.net/cover/5540/bionozos-of-soil-stm4t2sting-aoulim-hillooptera-by-hugh-975540.webp)
BIONOZOS of SOIL] STM4T2STING AOULIM HILLOOPTERA. by HUGH
BIONOZOS OF SOIL] STM4T2STING AOULIM HILLOOPTERA. by HUGH VICTOR DAMS, B.Sc. A Thesis submitted for the degree of Doctor of Philosophy in the Faculty of Science of the University of London Imperial College Field Station, Silwood Park, Sunninghill, September 1968 Ascut, Berkshire 2. ABSTRhCT Studios wore wade on the biolo:17 of a number of stem-nesting, aculeato Hymenoptera and their parasites, in particular on those nesting in Rubus fruticosus aai. (bramble), usirw, especially - as a means of obtaining nest material - trap-nests of bundles of stems of this plant. General observations and laboratory experiments 7ielded inform- ation for various species on nest architecture, prey, voltinism, dat- es of emergence, sex ratio, suporeedure, parwato/host relationships and other aspects of general aculeato bioloy. A laboratory culture of one species was established. The observed nest mortalities from various causes wore tabulated for each species. The total nest mortality was similar for most species, and indicated that the surv- ival of aculeate pro;7eny was hi;h compared to most insect species, but that the resultini; potential of a given female to produce large nufflbers of progeny was offset by the labour involved in makin;, nests and by competition for the limited number of available nestin..-sites. Population estimates of certain species wore also made, both by a combination of mark-recapture and trap-nest techniques, and by sam- pling the bramble bushes of the natural habitat. Some factors affect- ing the utilisation of nesting sites were also studied. Populations wore shown to be generally very low, and differences in the population size and in the dispersal of different species were detected. -

Nota Lepidopterologica
©Societas Europaea Lepidopterologica; download unter http://www.biodiversitylibrary.org/ und www.zobodat.at Nota lepid. 9 (3-4) : 225-233 ; 31.XII.1986 ISSN 0342-7536 Revue des Lépidoptères Gelechioidea de Grèce conservés au Musée Zoologique de Copenhague, y compris la description de la femelle de Ramphis libanoticus Riedl (Cosmopterigidae) Tadeusz Riedl Académie d'Éducation Physique, Laboratoire d'Écologie, Wiejska 1, 80-336 Gdansk, Pologne. Résumé L'auteur présente une liste de spécimens récoltés en Grèce méridionale (Pélopon- nèse) appartenant à quatre familles des Gelechioidea (Oecophoridae, Stathmopodi- dae, Momphidae, Cosmopterigidae) conservés au Musée Zoologique de Copenha- gue. Parmi 1 5 espèces, il est juste d'en énumérer quatre : Neomariania partinicensis (Rebel) qui n'était connu jusqu'à ce jour que de Sicile, ensuite Ramphis libanoticus Riedl, trouvé à Rhodes, dont la femelle et la nervation des ailes sont décrites, ainsi que Hodgesiella rebeli (Krone) et Eteobalea sumptuosella (Led.). La répartition et les dessins des genitalia de quelques espèces sont donnés. Monsieur Ole Karsholt (Copenhague) a eu la grande amabilité de me communiquer pour étude un abondant matériel de Gelechioidea, récolté en Grèce au cours des armées 1977-1984. Ce matériel constitue la propriété du Musée Zoologique (Zoologisk Museum) de Copenhague ; il contient 226 spécimens. La majorité d'entre eux a été récoltée par M. G. Christensen en Laconie, aux environs de Monemvasia (Péloponnèse mérid.), plus précisé- ment, à un distance de 5 km au sud ou 7 km au sud-ouest de cette ville. Plusieurs spécimens proviennent du Mont Taygète et des environs d'Athè- nes ; il semble juste de mentionner encore l'île de Rhodes, d'où proviennent 2 ex. -

Two New Fossil Species of Omaliinae from Baltic Amber
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arthropod Systematics and Phylogeny Jahr/Year: 2016 Band/Volume: 74 Autor(en)/Author(s): Zanetti Adriano, Perreau Michel, Solodovnikov Alexey Yurevitsh Artikel/Article: Two new fossil species of Omaliinae from Baltic amber (Coleoptera: Staphylinidae) and their significance for understanding the Eocene-Oligocene climate 53-64 74 (1): 53 – 64 14.6.2016 © Senckenberg Gesellschaft für Naturforschung, 2016. Two new fossil species of Omaliinae from Baltic amber (Coleoptera: Staphylinidae) and their significance for understanding the Eocene-Oligocene climate Adriano Zanetti 1, Michel Perreau *, 2 & Alexey Solodovnikov 3 1 Museo Civico di Storia Naturale, Lungadige Porta Vittoria 9, I-37129 Verona, Italy; Adriano Zanetti [[email protected]] — 2 Université Paris Diderot, Sorbonne Paris Cité, IUT Paris Diderot, case 7139, 5, rue Thomas Mann, F-75205 Paris cedex 13 France; Michel Perreau * [michel. [email protected]] — 3 Department of Entomology, Zoological Museum, Natural History Museum of Denmark, Universitetsparken 15, Copenhagen 2100, Denmark; Alexey Solodovnikov [[email protected]] — * Correspond ing author Accepted 23.ii.2016. Published online at www.senckenberg.de/arthropod-systematics on 03.vi.2016. Editor in charge: Christian Schmidt. Abstract Two fossil species, Paraphloeostiba electrica sp.n. and Phyllodrepa antiqua sp.n. (Staphylinidae, Omaliinae), are described from Baltic amber. Their external and relevant internal structures are illustrated using propagation phase contrast synchrotron microtomography. The palaeobiogeogaphy of the two genera, the thermophilous Paraphloeostiba, the temperate Phyllodrepa, as well as palaeoenvironment of the amber forest are discussed in light of the new findings. Key words Omaliini, Eusphalerini, synchrotron microtomography, temperate, thermophilous.