Descriptive Chemistry Assignment 4
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
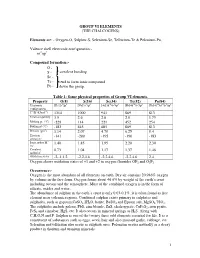
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

Black Phosphorus As a New Lubricant
Friction 6(1): 116–142 (2018) ISSN 2223-7690 https://doi.org/10.1007/s40544-018-0204-z CN 10-1237/TH RESEARCH ARTICLE Black phosphorus as a new lubricant Wei WANG, Guoxin XIE*, Jianbin LUO* State Key Laboratory of Tribology, Tsinghua University, Beijing 100084, China Received: 20 June 2017 / Revised: 18 October 2017 / Accepted: 07 December 2017 © The author(s) 2018. This article is published with open access at Springerlink.com Abstract: In recent years, a new 2D-layered material—black phosphorus (BP)—has been a rising star after the era of graphene owing to its high charge carrier mobility, tunable direct bandgap and unique in-plane anisotropic structure. With the development of the synthesis and modification methods of BP, its extensive applications, e.g., transistors, batteries and optoelectronics have emerged. In order to explore its full potential, research into the tribological properties of BP 2D-layered materials such as lubrication additives and fillers in self-lubricating composite materials would be not only of high scientific value but also of practical significance. In this work, recent advances on the friction and lubrication properties of BP nanosheets made by our group, including the micro-friction properties, the lubrication properties of BP nanosheets as water-based and oil-based lubrication additives, and the friction and wear of BP/PVDF composites will be presented. Finally, the future challenges and opportunities in the use of BP materials as lubricants will be discussed. Keywords: black phosphorus; two-dimensional (2D) material; lubricant additive; self-lubricating composite materials; friction 1 Introduction certain period owing to the finite lubricant thickness, and it can be easily affected by the environment. -

Amarsinha D. Nikam Vital Force Is Oxygen Extrait Du Livre Vital Force Is Oxygen De Amarsinha D
Amarsinha D. Nikam Vital Force is Oxygen Extrait du livre Vital Force is Oxygen de Amarsinha D. Nikam Éditeur : B. Jain http://www.editions-narayana.fr/b9138 Sur notre librairie en ligne vous trouverez un grand choix de livres d'homéopathie en français, anglais et allemand. Reproduction des extraits strictement interdite. Narayana Verlag GmbH, Blumenplatz 2, D-79400 Kandern, Allemagne Tel. +33 9 7044 6488 Email [email protected] http://www.editions-narayana.fr Oxygen Oxygen is derived from the Greek word, where oxys means acid, literally sharp from the taste of acids and genes means producer, literally begetter. It is the element with atomic number 8 and represented by the symbol 'O'. It is a highly reactive non-metallic period 2 element that readily forms compounds (notably oxides) with almost all other elements. Oxygen is the third most abundant element in the universe by mass after hydrogen and helium. Diatomic oxygen gas constitutes 21% of the volume of air. Water is the most familiar oxygen compound. OXYGEN HISTORY Oxygen makes up 21% of the atmosphere we breathe, but it was not discovered as a separate gas until the late i8th century. Oxygen was independently discovered by Carl Wilhelm Scheele, in Uppsala in 1773 or earlier and by Joseph Priestley in Wiltshire, in 1774. The name oxygen was coined in 1777 by Antoine Lavoisier. Although oxygen plays a life-supporting role, it took Narayana Verlag, 79400 Kandern Tel.: 0049 7626 974 970 0 Excerpt from Dr. Amarsinha D.Nikam: Vital Force is Oxygen about 150 years for the gas to be used in a proper manner for patients. -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

Environment and Ecology BHM 403 Techno India
Environment and Ecology BHM 403 Techno India ENVIRONMENT AND ECOLOGY BHM 403 (2008 -2017) PREPARED BY ANIS CHATTOPADHYAY ASST. PROFESSOR TECHNO INDIA EM-4/1, SECTOR –V, SALT LAKE KOLKATA -700091 1 Environment and Ecology BHM 403 Techno India 2008 GROUP – A ( Multiple Choice Type Questions ) 1. Choose correct answer from the given alternatives in each of the following questions : 10x1 = 10 i) Environmental Studies involve studies of a) evolution of life b) all aspects of human environment c) nitrogen cycle d) oxygen cycle. b ii) "Itai itai' disease is caused by a) Zinc b) Cadmium c) Mercury d) Iron. b iii) El Nino starts from a) Mediterranean coast b) Chinese coast c) South American cost d) Indian cost. C iv) The Greenhouse effect is due to a) Carbon dioxide, water vapour, methane and chlorofluorocarbons b) Nitrogen oxide c) Sulphur oxide d) Carbon monoxide. A v) The Ganga pollution is due to dumping of a) domestic and industrial sewages b) waste from forest c) food waste d) hospital waste. A vi) Biotic factor of ecosystem is a) Solar energy b) Temperature c) Soil d) Plants and animals. D vii) Medha Patkar is involved in a) Chipko movement b) Silent Valley movement c) Narmada Bachao movement d) none of these. C viii) The "Kyoto Protocol" is related with a) air pollution b) noise pollution c) water pollution d) none of these. A ix) BOD stands as a) Biochemical Oxygen Demand b) Biological Oxygen Demand c) Biggest Oxygen Demand d) Blown Out Dose. B x) The protective shield for life on earth is a) Carbon dioxide b) Ozone c) Oxygen d) Hydrogen. -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1. -

Introduction to Phase Diagrams*
ASM Handbook, Volume 3, Alloy Phase Diagrams Copyright # 2016 ASM InternationalW H. Okamoto, M.E. Schlesinger and E.M. Mueller, editors All rights reserved asminternational.org Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a a system with varying composition of two com- Nevertheless, phase diagrams are instrumental physically homogeneous state of matter with a ponents. While other extensive and intensive in predicting phase transformations and their given chemical composition and arrangement properties influence the phase structure, materi- resulting microstructures. True equilibrium is, of atoms. The simplest examples are the three als scientists typically hold these properties con- of course, rarely attained by metals and alloys states of matter (solid, liquid, or gas) of a pure stant for practical ease of use and interpretation. in the course of ordinary manufacture and appli- element. The solid, liquid, and gas states of a Phase diagrams are usually constructed with a cation. Rates of heating and cooling are usually pure element obviously have the same chemical constant pressure of one atmosphere. too fast, times of heat treatment too short, and composition, but each phase is obviously distinct Phase diagrams are useful graphical representa- phase changes too sluggish for the ultimate equi- physically due to differences in the bonding and tions that show the phases in equilibrium present librium state to be reached. However, any change arrangement of atoms. in the system at various specified compositions, that does occur must constitute an adjustment Some pure elements (such as iron and tita- temperatures, and pressures. It should be recog- toward equilibrium. Hence, the direction of nium) are also allotropic, which means that the nized that phase diagrams represent equilibrium change can be ascertained from the phase dia- crystal structure of the solid phase changes with conditions for an alloy, which means that very gram, and a wealth of experience is available to temperature and pressure. -

Unified Science Volume 1: Mathematics, Physics and Chemistry
UNIFIED SCIENCE VOLUME 1: MATHEMATICS, PHYSICS AND CHEMISTRY RICHARD L. LEWIS, PHD [email protected] UNIFICATION THOUGHT INSTITUTE JAPAN • KOREA • USA © 2013 Thank you True Parents CONTENTS Unified Science!.............................................................1 The Abstract Realm!......................................................3 Linear Extension!..........................................................................3 The integers!.................................................................................3 Passive and active!.......................................................................5 Primes!..........................................................................................7 Infinity of infinities!.........................................................................8 Transcendental numbers!............................................................10 Distribution of the primes!...........................................................12 Circular Rotation!.......................................................................13 The (co)sine function!..................................................................14 Waves!.........................................................................................16 Complex numbers!.....................................................................23 The Rotation Operator!................................................................24 Harmonic Primes!........................................................................29 Abstract Hierarchy!....................................................................31 -

Download (4Mb)
Original citation: Zhenqing, Li, He, Chaoyu, Ouyang, Tao, Zhang, Chunxiao, Tang, Chao, Roemer, Rudolf A. and Zhong, Jianxin (2018) New allotropes of phosphorene with remarkable stability and intrinsic piezoelectricity. Physical Review Applied, 9 . 044032. doi:10.1103/PhysRevApplied.9.044032 Permanent WRAP URL: http://wrap.warwick.ac.uk/101768 Copyright and reuse: The Warwick Research Archive Portal (WRAP) makes this work by researchers of the University of Warwick available open access under the following conditions. Copyright © and all moral rights to the version of the paper presented here belong to the individual author(s) and/or other copyright owners. To the extent reasonable and practicable the material made available in WRAP has been checked for eligibility before being made available. Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. Publisher statement: © 2018 American Physical Society Published version: https://doi.org/10.1103/PhysRevApplied.9.044032 A note on versions: The version presented here may differ from the published version or, version of record, if you wish to cite this item you are advised to consult the publisher’s version. Please see the ‘permanent WRAP url’ above for details on accessing the published version and note that access may require a subscription. For more information, please contact the WRAP Team at: [email protected] warwick.ac.uk/lib-publications New allotropes of phosphorene with remarkable stability and intrinsic piezoelectricity Zhenqing Li,1, 2 Chaoyu He,1, 2, ∗ Tao Ouyang,1, 2 Chunxiao Zhang,1, 2 Chao Tang,1, 2, y Rudolf A. -

Recent Advances in Synthesis, Properties, and Applications of Phosphorene
www.nature.com/npj2dmaterials REVIEW ARTICLE OPEN Recent advances in synthesis, properties, and applications of phosphorene Meysam Akhtar1,2, George Anderson1, Rong Zhao1, Adel Alruqi1, Joanna E. Mroczkowska3, Gamini Sumanasekera1,2 and Jacek B. Jasinski2 Since its first fabrication by exfoliation in 2014, phosphorene has been the focus of rapidly expanding research activities. The number of phosphorene publications has been increasing at a rate exceeding that of other two-dimensional materials. This tremendous level of excitement arises from the unique properties of phosphorene, including its puckered layer structure. With its widely tunable band gap, strong in-plane anisotropy, and high carrier mobility, phosphorene is at the center of numerous fundamental studies and applications spanning from electronic, optoelectronic, and spintronic devices to sensors, actuators, and thermoelectrics to energy conversion, and storage devices. Here, we review the most significant recent studies in the field of phosphorene research and technology. Our focus is on the synthesis and layer number determination, anisotropic properties, tuning of the band gap and related properties, strain engineering, and applications in electronics, thermoelectrics, and energy storage. The current needs and likely future research directions for phosphorene are also discussed. npj 2D Materials and Applications (2017) 1:5 ; doi:10.1038/s41699-017-0007-5 INTRODUCTION properties, including high carrier mobility, ultrahigh surface area, Since the discovery of graphene in 2004 (ref. 1), there has been a excellent thermal conductivity, and quantum confinement effect 9 quest for new two-dimensional (2D) materials aimed at fully have been well documented. However, the lack of band gap is a exploring new fundamental phenomena stemming from quantum serious limitation for the use of graphene in electronic devices. -

2. Allotropy in Carbon the Property Due to Which an Element Exists In
SUBJECT-CHEMISTRY DATE- 22/11/2020 CLASS- X TOPICS- Chap. 4 Carbon and its Compounds Carbon is one of the most essential components of living organisms. There are two stable isotopes of carbon C-12 and C-13. After these two one more isotope of carbon is present C-14. Carbon is used for radiocarbon dating One of the most amazing properties of carbon is its ability to make long carbon chains and rings. This property of carbon is known as catenation. Carbon has many special abilities out of all one unique ability is that carbon forms double or triple bonds with itself and with other electronegative atoms like oxygen and nitrogen. These two properties of carbon i.e catenation and multiple bond formation, it has the number of allotropic forms. Allotrope is nothing but the existence of an element in many forms which will have different physical property but will have similar chemical properties and its forms are called allotropes of allotropic forms. Allotropes are defined as the two or more physical forms of one element. These allotropes are all based on carbon atoms but exhibit different physical properties, especially with regard to hardness. The two common, crystalline allotropes of carbon are diamond and graphite. Carbon shows allotropy because it exists in different forms of carbon. Though these allotropes of carbon have a different crystal structure and different physical properties, their chemical properties are the same and show similar chemical properties. Both diamond and graphite have symbol C. Both give off carbon dioxide when strongly heated in the presence of oxygen. -

Download SCOPE Newsletter
With participation of the European Commission: DG GROW and Joint Research Centre Summary of joint European Commission – ESPP webinar th on P4 (phosphorus) Critical Raw Material, 9 July 2020 This ESPP SCOPE Newsletter summarises discussions at the expert webinar jointly organised by ESPP and the European Commission on 9th July, with participation of Contents the leading companies using phosphorus (P4) or its derivatives in Europe, with additional input from the The EU Critical Raw Materials: “Phosphate Rock” and “Phosphorus”........................................................................... 2 independent industry expert Willem Schipper. The content (Fig. i) Overview numbers: from phosphate rock to P4 / P4-derivatives below has been seen by the participants but does not and applications .................................................................................. 3 represent their position, it represents ESPP’s own How is P4 produced? the phosphorus furnace .............. 4 assessment. Photo: P4 furnace ................................................................................ 4 Schematic: P4 manufacture process (simplified) ................................. 4 This webinar aimed to provide expert input to the European Which chemicals critically depend on P4 / P4 derivatives? . 5 Commission’s MSA (Material System Analysis) of the EU dependency on P4 concerns many industry sectors . 5 specific industrial form of “Phosphorus”, P4, as a Critical (Fig. ii) Production via wet-acid or via P4 / P4 derivatives / ‘thermal’ Raw