Atomic Weights of the Elements 2013 (IUPAC Technical Report)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

Package 'Ciaawconsensus'
Package ‘CIAAWconsensus’ September 19, 2018 Type Package Title Isotope Ratio Meta-Analysis Version 1.3 Author Juris Meija and Antonio Possolo Maintainer Juris Meija <[email protected]> Description Calculation of consensus values for atomic weights, isotope amount ratios, and iso- topic abundances with the associated uncertainties using multivariate meta-regression ap- proach for consensus building. License Unlimited LazyData yes Imports mvtnorm, stringr, numDeriv, stats, Matrix NeedsCompilation no Repository CRAN Date/Publication 2018-09-19 13:30:12 UTC R topics documented: abundances2ratios . .2 at.weight . .3 ciaaw.mass.2003 . .4 ciaaw.mass.2012 . .5 ciaaw.mass.2016 . .6 iridium.data . .6 mmm ............................................7 normalize.ratios . .8 platinum.data . .9 Index 10 1 2 abundances2ratios abundances2ratios Isotope ratios of a chemical element from isotopic abundances Description This function calculates the isotope ratios of a chemical element from the given isotopic abundances and their uncertainties. The uncertainty evaluation is done using the propagation of uncertainty and the missing correlations between the isotopic abundances are reconstructed using Monte Carlo methods. Usage abundances2ratios(x, ux, ref=1, iterations=1e4) Arguments x A vector of isotopic abundances of an element ux Standard uncertainties of x ref Index to specify the desired reference isotope for isotope amount ratios iterations Number of iterations for isotopic abundance correlation mapping Details Situations are often encountered where isotopic abundances are reported but not the isotope ratios. In such cases we reconstruct the isotope ratios that are consistent with the abundances and their uncertainties. Given only the abundances and their uncertainties, for elements with four or more isotopes one cannot unambiguously infer the uncertainties of the ratios due to the unknown correla- tions between isotopic abundances. -

NOBELIUM Element Symbol: No Atomic Number: 102
NOBELIUM Element Symbol: No Atomic Number: 102 An initiative of IYC 2011 brought to you by the RACI KERRY LAMB www.raci.org.au NOBELIUM Element symbol: No Atomic number: 102 The credit for discovering Nobelium was disputed with 3 different research teams claiming the discovery. While the first claim dates back to 1957, it was not until 1992 that the International Union of Pure and Applied Chemistry credited the discovery to a research team from Dubna in Russia for work they did in 1966. The element was named Nobelium in 1957 by the first of its claimed discoverers (the Nobel Institute in Sweden). It was named after Alfred Nobel, a Swedish chemist who invented dynamite, held more than 350 patents and bequeathed his fortune to the establishment of the Nobel Prizes. Nobelium is a synthetic element and does not occur in nature and has no known uses other than in scientific research as only tiny amounts of the element have ever been produced. Nobelium is radioactive and most likely metallic. The appearance and properties of Nobelium are unknown as insufficient amounts of the element have been produced. Nobelium is made by the bombardment of curium (Cm) with carbon nuclei. Its most stable isotope, 259No, has a half-life of 58 minutes and decays to Fermium (255Fm) through alpha decay or to Mendelevium (259Md) through electron capture. Provided by the element sponsor Freehills Patent and Trade Mark Attorneys ARTISTS DESCRIPTION I wanted to depict Alfred Nobel, the namesake of Nobelium, as a resolute young man, wearing the Laurel wreath which is the symbol of victory. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Properties of Carbon the Atomic Element Carbon Has Very Diverse
Properties of Carbon The atomic element carbon has very diverse physical and chemical properties due to the nature of its bonding and atomic arrangement. fig. 1 Allotropes of Carbon Some allotropes of carbon: (a) diamond, (b) graphite, (c) lonsdaleite, (d–f) fullerenes (C60, C540, C70), (g) amorphous carbon, and (h) carbon nanotube. Carbon has several allotropes, or different forms in which it can exist. These allotropes include graphite and diamond, whose properties span a range of extremes. Despite carbon's ability to make 4 bonds and its presence in many compounds, it is highly unreactive under normal conditions. Carbon exists in 2 main isotopes: 12C and 13C. There are many other known isotopes, but they tend to be short-lived and have extremely short half-lives. Allotropes The different forms of a chemical element. Cabon is the chemical element with the symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon has 6 protons and 6 Source URL: https://www.boundless.com/chemistry/nonmetallic-elements/carbon/properties-carbon/ Saylor URL: http://www.saylor.org/courses/chem102#6.1 Attributed to: Boundless www.saylor.org Page 1 of 2 neutrons, and has a standard atomic weight of 12.0107 amu. Its electron configuration is denoted as 1s22s22p2. It is a solid, and sublimes at 3,642 °C. It's oxidation state ranges from 4 to -4, and it has an electronegativity rating of 2.55 on the Pauling scale. Carbon has several allotropes, or different forms in which it exists. -
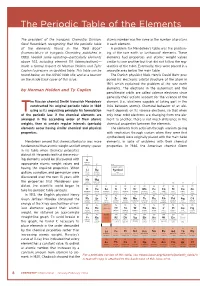
The Periodic Table of the Elements
The Periodic Table of the Elements The president of the Inorganic Chemistry Division, atomic number was the same as the number of protons Gerd Rosenblatt, recognizing that the periodic table in each element. of the elements found in the “Red Book” A problem for Mendeleev’s table was the position- (Nomenclature of Inorganic Chemistry, published in ing of the rare earth or lanthanoid* elements. These 1985) needed some updating—particularly elements elements had properties and atomic weight values above 103, including element 110 (darmstadtium)— similar to one another but that did not follow the reg- made a formal request to Norman Holden and Tyler ularities of the table. Eventually, they were placed in a Coplen to prepare an updated table. This table can be separate area below the main table. found below, on the IUPAC Web site, and as a tear-off The Danish physicist Niels Henrik David Bohr pro- on the inside back cover of this issue. posed his electronic orbital structure of the atom in 1921, which explained the problem of the rare earth by Norman Holden and Ty Coplen elements. The electrons in the outermost and the penultimate orbits are called valence electrons since generally their actions account for the valence of the he Russian chemist Dmitri Ivanovich Mendeleev element (i.e., electrons capable of taking part in the constructed his original periodic table in 1869 links between atoms). Chemical behavior of an ele- Tusing as its organizing principle his formulation ment depends on its valence electrons, so that when of the periodic law: if the chemical elements are only inner orbit electrons are changing from one ele- arranged in the ascending order of their atomic ment to another, there is not much difference in the weights, then at certain regular intervals (periods) chemical properties between the elements. -

Contents Contents
Contents CHEMISTRY International January-March 2019 Volume 41 No. 1 Introduction The International Year of the Periodic Table 2019 2 by Jan Reedijk and Natalia Tarasova Features IUPAC and the Periodic Table by G.J. Leigh 6 Criteria for New Element Discovery: Providing Assurance 10 in a Field of Allure and Romance by Sigurd Hofmann Looking Backwards and Forwards at the Development 16 of the Periodic Table by Eric Scerri Isotopic Abundances and Atomic Weights: History of IUPAC 21 Commission II.1 in the Service of Chemistry by John R. De Laeter IUPAC Commission II.1 Today by Juris Meija 24 Isotopes Matter by Norman E. Holden, Tyler B. Coplen, and Peter Mahaffy 27 The New SI: The International System of Units is Getting 32 a Makeover by Ian Mills and Roberto Marquardt IUPAC Wire Election of IUPAC Officers and Bureau Members 36 Juris Meija Made the Top 40 Under 40 37 C. Oliver Kappe is Awarded the 2018 IUPAC-ThalesNano Prize 37 for Flow Chemistry OPCW to Further Enhance Contributions to United Nations’ 38 Sustainable Development Goals 1001 Inventions: Journeys from Alchemy to Chemistry 38 Stamps International 39 Project Place Critical evaluation of homogeneous equilibrium and solubility 40 constants of gadolinium in environmental and biological- relevant conditions 23rd UNESCO/IUPAC Postgraduate Course in Polymer Science 40 Guidance for the Compilation, Critical Evaluation and 40 Dissemination of Chemical Data Making an imPACt On the Discovery of New Elements (IUPAC/IUPAP 42 Provisional Report) IUPAC Periodic Table of the Elements and Isotopes -

Diffusion of Carbon in Niobium and Molybdenum
Materials Transactions, Vol. 55, No. 12 (2014) pp. 1786 to 1791 ©2014 The Japan Institute of Metals and Materials Diffusion of Carbon in Niobium and Molybdenum Jun-ichi Imai1, Osamu Taguchi2,+, Gyanendra Prasad Tiwari3 and Yoshiaki Iijima1 1Department of Materials Science, Graduate School of Engineering, Tohoku University, Sendai 980-8579, Japan 2Department of Materials Science and Engineering, Miyagi National College of Technology, Natori 981-1239, Japan 3Department of Information Technology, Ramrao Adik Institute of Technology, Vidya Nagri, Nerul, Navi Mumbai 400709, India Diffusion coefficients of carbon in niobium and molybdenum have been determined by the residual activity method with radioactive tracer 14C in the temperature ranges between 1168 and 1567 K for niobium and between 1271 and 1669 K for molybdenum. The temperature dependences of the diffusion coefficient of carbon in niobium and molybdenum are expressed by D/m2 s¹1 = 2.2 © 10¹6 exp(¹152 kJ mol¹1/ RT) and D/m2 s¹1 = 5.2 © 10¹6 exp(¹163 kJ mol¹1/RT), respectively. Since the solubility of carbon in molybdenum is very small, the diffusion of carbon in molybdenum is strongly influenced by carbide precipitation at lower temperatures. [doi:10.2320/matertrans.M2014277] (Received July 31, 2014; Accepted September 30, 2014; Published November 8, 2014) Keywords: carbon diffusion, niobium, molybdenum, carbon solubility, precipitate effect 1. Introduction 2. Experimental Procedure Iron, nickel and cobalt based superalloys appear to have 2.1 Material achieved full potential in relation to their use as structural Niobium metal rod arc-melted and machined to 12.5 mm in materials for corrosive environments as well as high diameter was supplied by Materials Research Corporation, temperatures.1) The strength of these alloys comes partly USA. -

Concurrent Reduction and Distillation; an Improved Technique for the Recovery and Chemical Refinement of the Isotopes of Cadmium and Zinc*
If I CONCURRENT REDUCTION AND DISTILLATION; AN IMPROVED TECHNIQUE FOR THE RECOVERY AND CHEMICAL REFINEMENT OF THE ISOTOPES OF CADMIUM AND ZINC* H. H. Cardill L. E. McBride LOHF-821046—2 E. W. McDaniel DE83 001931 Operations Division Oak Ridge National Laboratory Oak Ridge, Tennessee 3 7830 .DISCLAIMER • s prepared es an accouni oi WOTk sconsQ'ed by an agency of itie United Slates Cover r "iiit?3 Stilt 65 Gov(?fnft»gn ^ ri(jt ^nv aQcncy Tllf GO* ^or rlny O^ tt^^ir ^rnployut^. TigV MASTER lostifl. ion, apod'aiuj pioiJut;. <y vned nghtv Reference hcein xc any v>t^-'T*t. ^ Dv ^.r^de narno, tf^Oernafk^ mir'ij'iJClureT. or otrierw «d <Jo*^ •nenaalion. or liivONng bv 'Vhe United _^_ . vs and opinions of authors enp'«i«] herein do noi IOM t' irip UnitK* Stales Govefnrneni c a^v agency For presentation at the 11th World Conference of the International Nuclear Target Development Society, October 6-8, 1982, Seattle, Washington. By acceptance of this article, the publisher or recipient acknowledge* the U.S. Govarnment's right to retain a nonaxclusiva, royalty-frtta license in and to any copyright covering tha article. NOTICE P0T09_0£-Jfns,J^SPQRT_JfgE_Il,LESIBLE. I has been reproauce.1 from the best available aopy to pensit the broadest possible avail- ability* *Research sponsored by the Office of Basic Energy Science, U.S. Department of Energy under contract W-7405-eng-26 with the Union Carbide Co rpcration,, DISTRIBUTION OF THIS DOCUMEHT 18 UNLIMITED ' CONCURRENT REDUCTION AND DISTILLATION - AY. IMPROVED TECHNIQUE FOR THE RECOVERY AND CHEMICAL REFINEMENT OF THE ISOTOPES OF CADMIUM AND ZINC H. -

KULAGE-DISSERTATION-2013.Pdf
ENCRYPTED NANOPARTICLES FOR SECURITY, COUNTER-INDUSTRIAL ESPIONAGE, AND COUNTERFEITING A Dissertation by ZACHARY ANDREW KULAGE Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Chair of Committee, William S. Charlton Committee Members, Sean M. McDeavitt John W. Poston, Sr. Charles M. Folden III Head of Department, Yassin A. Hassan December 2013 Major Subject: Nuclear Engineering Copyright 2013 Zachary Andrew Kulage ABSTRACT Acts of terrorism and the use of explosives raise concerns about what can be done to prevent such acts. From stopping such a threat to preventing the illicit counterfeiting and smuggling of sensitive and proprietary goods, identification tag- gant technology can be applied to identify and interdict materials of a dubious na- ture. This cost effective approach to tag and track materials will find application in both the government and private sectors, particularly in the prevention of corporate espionage. A taggant system was created based on the modification of the natural isotopic vector of cadmium and tellurium to create artificial, unique, and robust identification capabilities. A proof of concept trial was undertaken to ascertain the effectiveness, reliability, and integrity of a system. Stable isotopes were mixed in various combi- nations to alter the isotopic vector and then neutron activation analysis techniques were used to evaluate the quality of the taggants. Two different neutron activation analysis facilities of differing capabilities were employed to test the limitations of the method in detection of trace amounts of taggant material. Testing of the taggant system found that solid phase taggants were capable of producing around 14,000 unique taggants for under $250 each while liquid phase taggants were capable of producing around 2,000 unique taggants for under $50 each. -

Neutron Capture Cross Sections of Cadmium Isotopes
Neutron Capture Cross Sections of Cadmium Isotopes By Allison Gicking A thesis submitted to Oregon State University In partial fulfillment of the requirements for the degree of Bachelor of Science Presented June 8, 2011 Commencement June 17, 2012 Abstract The neutron capture cross sections of 106Cd, 108Cd, 110Cd, 112Cd, 114Cd and 116Cd were determined in the present project. Four different OSU TRIGA reactor facilities were used to produce redundancy in the results and to measure the thermal cross section and resonance integral separately. When the present values were compared with previously measured values, the differences were mostly due to the kind of detector used or whether or not the samples were natural cadmium. Some of the isotopes did not have any previously measured values, and in that case, new information about the cross sections of those cadmium isotopes has been provided. Table of Contents I. Introduction………………………………………………………………….…….…1 II. Theory………………………………………………………………………...…...…3 1. Neutron Capture…………………………………………………….….……3 2. Resonance Integral vs. Effective Thermal Cross Section…………...………5 3. Derivation of the Activity Equations…………………………………....…..8 III. Methods………………………………………………………….................…...…...12 1. Irradiation of the Samples………………………………………….….....…12 2. Sample Preparation and Parameters………………..………...………..……16 3. Efficiency Calibration of Detectors…………………………..………....…..18 4. Data Analysis…………………………………...…….………………...…..19 5. Absorption by 113Cd……………………………………...……...….………20 IV. Results………………………………………………….……………..……….…….22 -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table.